Co-amorphous powder for treating asthmatic lung diseases and preparation method thereof
A lung disease, amorphous technology, applied in the field of crystal form and medicine, can solve problems such as side effects, high local concentration, hypokalemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
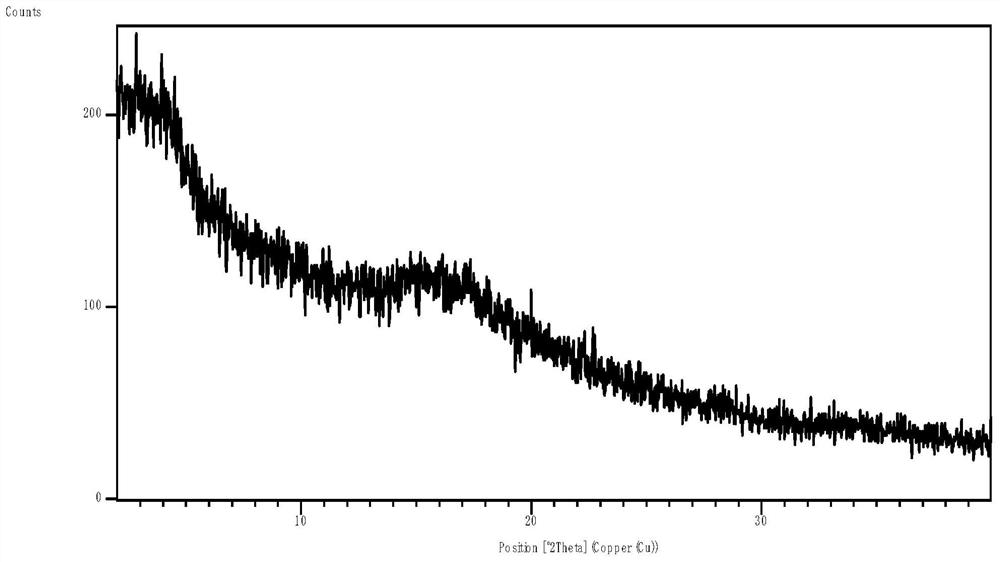
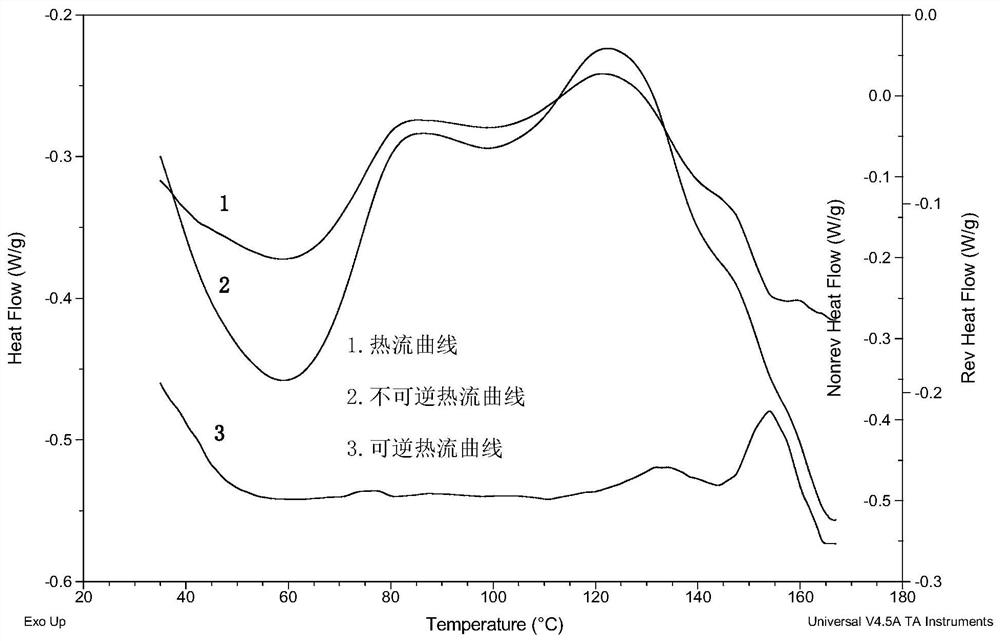
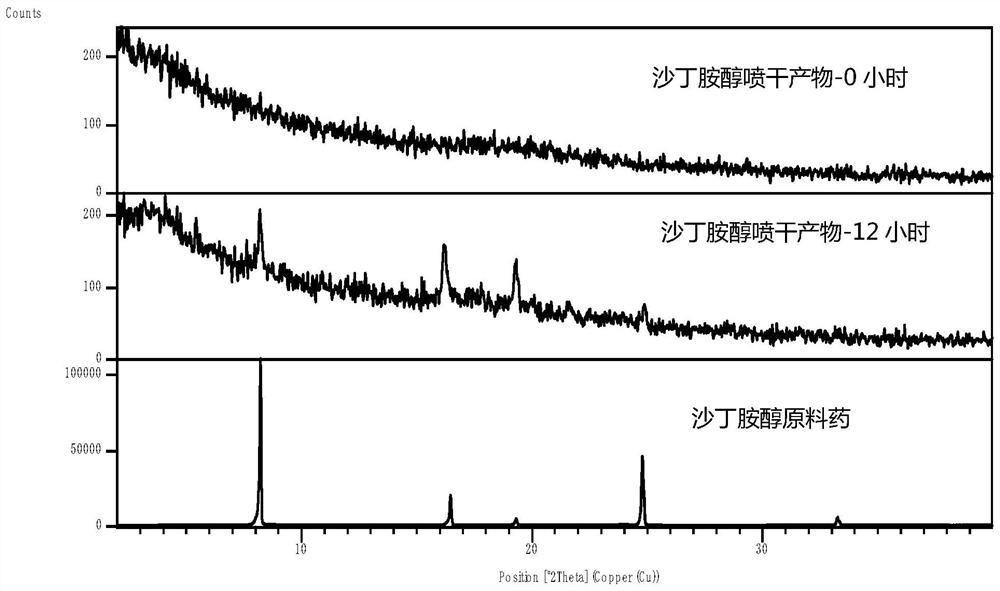
[0062] Salbutamol, various insoluble corticosteroids, and carriers were characterized by XRPD, DSC, and Raman, respectively.
[0063] 1. The ratio of albuterol to budesonide is 1:1, 1:2, 2:1. First, the co-crystal screening of the two active drugs was carried out, and the methods of suspension crystallization, cooling crystallization, and volatile crystallization were used respectively. The XRPD diffraction patterns showed a physical mixture of the two drugs, and it was difficult to obtain the co-crystal product of the two drugs.
[0064] 2. Using the ball milling method, ball milled albuterol and budesonide respectively. It was found that salbutamol could be milled for 40 minutes to obtain an amorphous form. However, crystal transformation occurred after 1.5 days at room temperature, indicating poor stability; budesonide still existed after ball milling for 60 minutes. Diffraction peaks of the crystal form of the starting material. Mix budesonide with different carriers resp...
Embodiment 2
[0108] Get 55.58mg salbutamol, 100mg budesonide, after being dissolved in 25ml 95% (v / v) ethanol, stir for 1 hour, then filter and get the clear liquid and spray dry, the condition is: inlet temperature 70 ℃, outlet temperature 50 ℃, cooling temperature : -17°C, injection speed 5%-7% (1.5ml / min-2.1ml / min), gas delivery rate 100%, constant temperature stirring at 37°C during the process until the liquid is sprayed out, and the obtained product is sealed and stored immediately.
Embodiment 3
[0110] Dissolve 30.24mg of serine in 3ml of purified water to prepare a serine solution. Weigh 60.11mg of albuterol and 109.82mg of budesonide, dissolve in 30ml of absolute ethanol, then add serine solution, stir for 60min to mix well, then ultrasonicate at 40KHz, 40℃ for 1 hour and then spray dry. The conditions are: inlet temperature 70°C, outlet temperature 48°C, cooling temperature: -17°C, sample injection speed 10%-12% (3ml / min-3.6ml / min), air supply 100%, and 37°C during the process Stir at constant temperature until the liquid is sprayed out, thereby obtaining a co-amorphous powder (ternary co-amorphous substance) for treating asthmatic lung diseases, which is immediately sealed and stored.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com