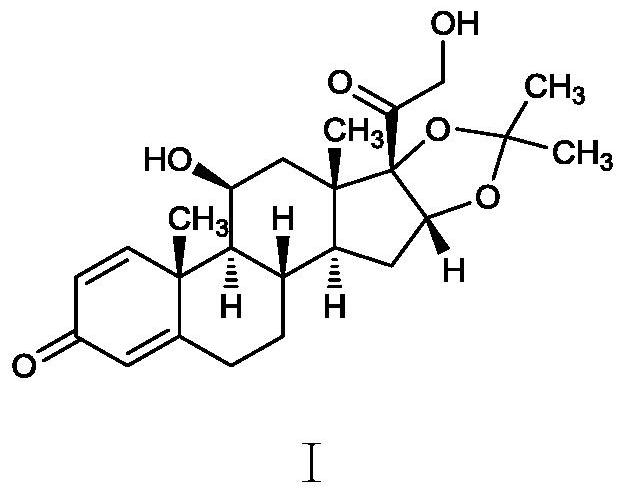
Method for synthesizing desonide impurity
A synthesis method and technology of mixed solution, applied in steroids, organic chemistry and other directions, can solve problems such as complicated operation, and achieve the effects of simple operation, favorable for industrial production and mild method conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
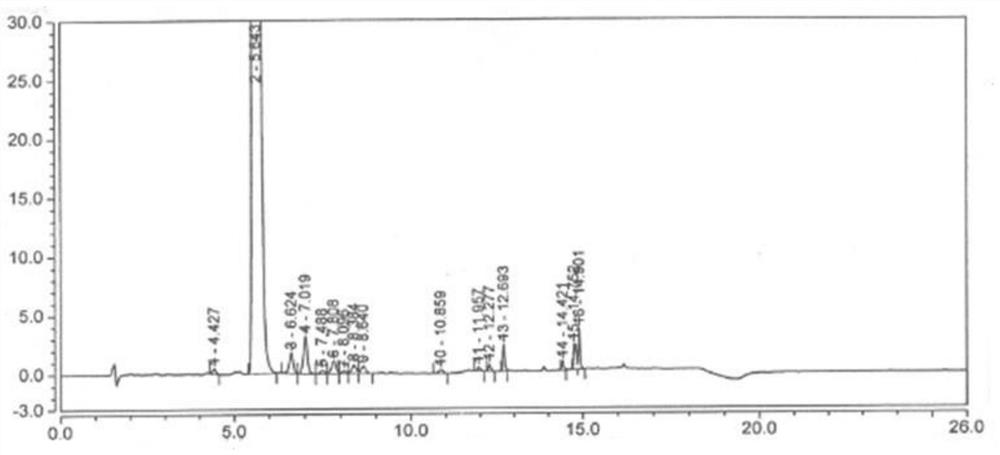
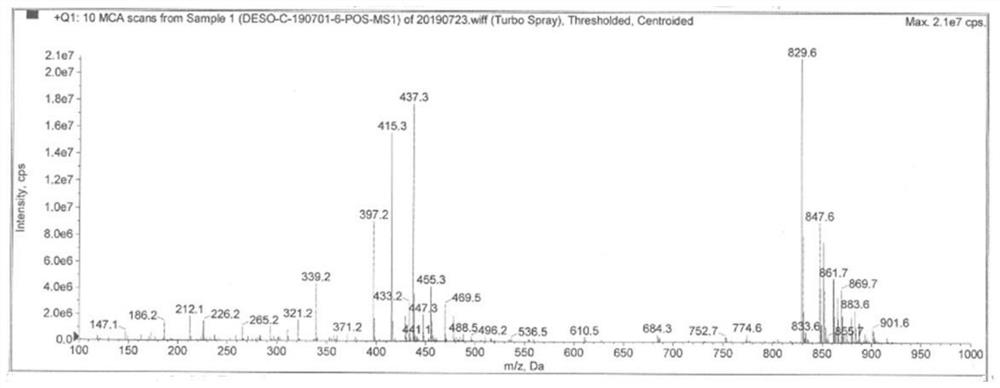
Image
Examples
Embodiment 1
[0037] Add 2g of desonide and 0.5g of copper acetate into a 250ml three-neck flask, fully dissolve in 150ml of methanol to form a solution, when the solution is heated to 30°C, blow air into the solution to react to obtain a reaction solution, the reaction solution is pale yellow, and the reaction After 2h, TLC (CH2Cl2:CH3OH 15:1) test showed that the reaction of the raw materials was complete, and the reaction was stopped; 150ml of water was added to the reaction solution, and the aqueous layer was extracted twice with 250ml of dichloromethane respectively, and the organic layers extracted twice were combined and washed with water. The organic layer was concentrated under reduced pressure to dryness to obtain a white solid.
[0038] Add 250ml of acetonitrile to the white solid, stir to dissolve, add 80ml of water and 2 drops of phosphoric acid, react at 40-50°C for 1 hour, the product monitored by HPLC is 96%, stop the reaction; evaporate the acetonitrile under reduced pressur...
Embodiment 2
[0045] Add 2g of desonide and 0.2g of copper acetate to a 250ml three-neck flask, fully dissolve in 150ml of methanol to form a solution, and when the solution is heated to 30°C, blow air into the solution to react to obtain a reaction solution. After 2h, TLC (CH2Cl2:CH3OH 15:1) test showed that the reaction of the raw materials was complete, and the reaction was stopped; 150ml of water was added to the reaction solution, and the aqueous layer was extracted twice with 250ml of dichloromethane respectively, and the organic layers extracted twice were combined and washed with water. The organic layer was concentrated under reduced pressure to dryness to obtain a white solid.
[0046] Add 150ml of acetone to the white solid, stir to dissolve, add 80ml of water and 2 drops of phosphoric acid, react at 40-50°C for 1 hour, the product monitored by HPLC is 94%, and the reaction is stopped; the acetone is evaporated under reduced pressure, and 250ml of dichloro Extracted twice with me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com