3-trifluoromethyl quinoxalinone compound preparation method
A technology of trifluoromethylquinoxalinone and quinoxalinone, which is applied in the field of preparation of 3-trifluoromethylquinoxalinone compounds, can solve problems such as difficult operation and expensive hexafluoropropylene oxide, and achieve The effect of mild conditions and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
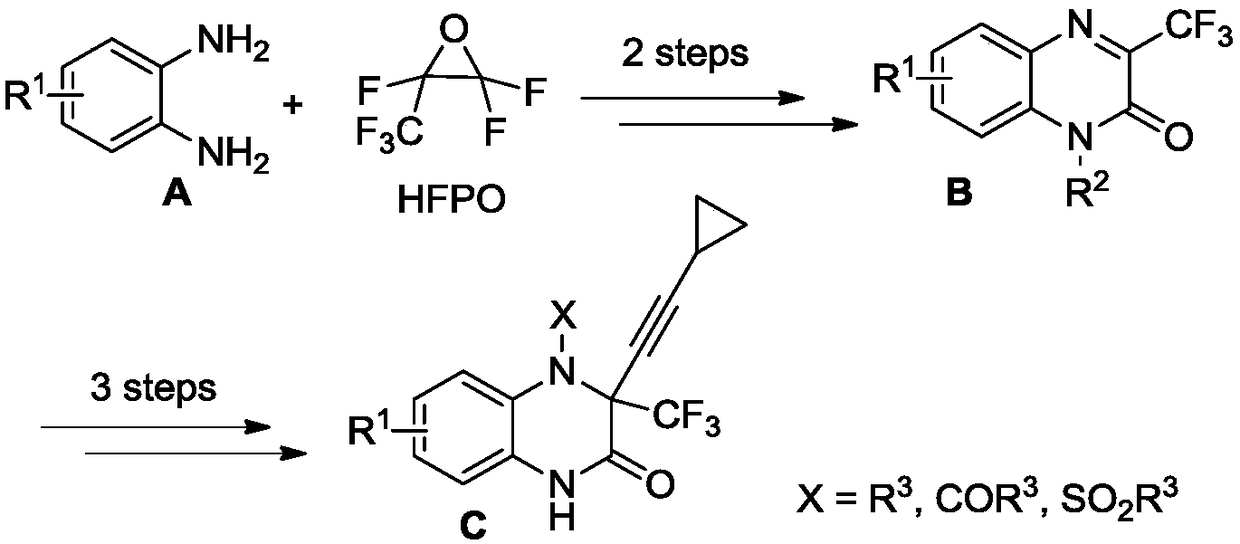
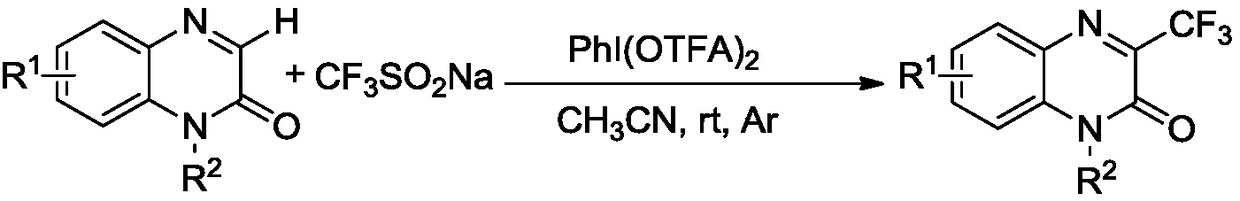
[0026] Add the substrate 1-methylquinoxalin-2(1H)-one to a 15 ml reaction tube (48.0 mg, 0.3 mmol, the substituent R on the structural formula 1 is methyl, R 2 is a hydrogen atom), sodium trifluoromethanesulfinate (CF 3 SO 2 Na, 140.5 mg, 0.9 mmol), and the oxidant iodobenzene bistrifluoroacetate (387.0 mg, 0.9 mmol) was added, the oil pump was evacuated, argon was filled, and repeated 3 times, 3 ml of acetonitrile was added with a syringe. The mixture was stirred at room temperature under argon for 12 hours. Thin-layer chromatography detects that the reaction is substantially complete, and the solvent acetonitrile is removed by rotary evaporation, and the column chromatography (ethyl acetate / petroleum ether=1 / 3) is directly used to obtain the product 1-methyl-3-trifluoromethylquinoxaline- 2(1H)-keto 54.8 mg, total yield 80%.
[0027] The hydrogen nuclear magnetic resonance spectrum data of gained product is 1 H NMR (400MHz, CDCl 3 )δ: 8.05(dd, J=1.2Hz, J=44Hz, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com