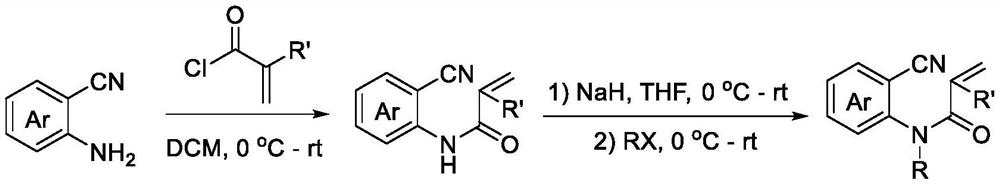
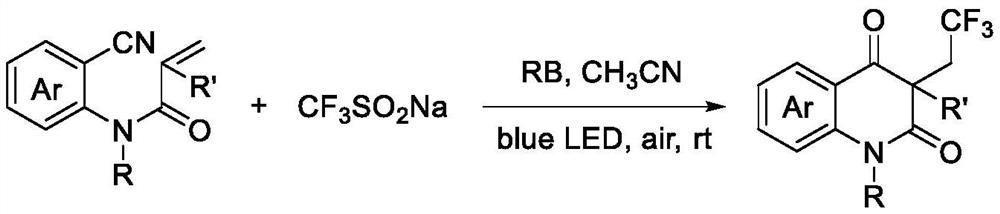
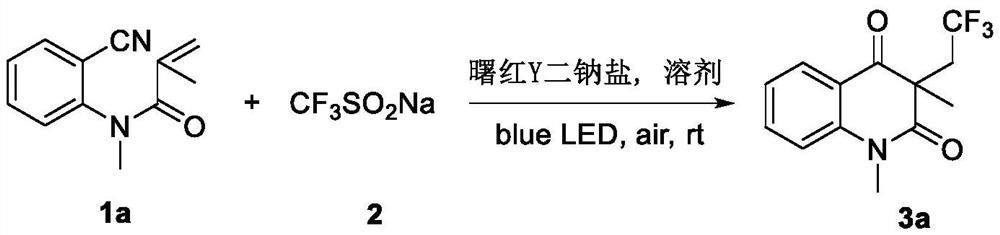
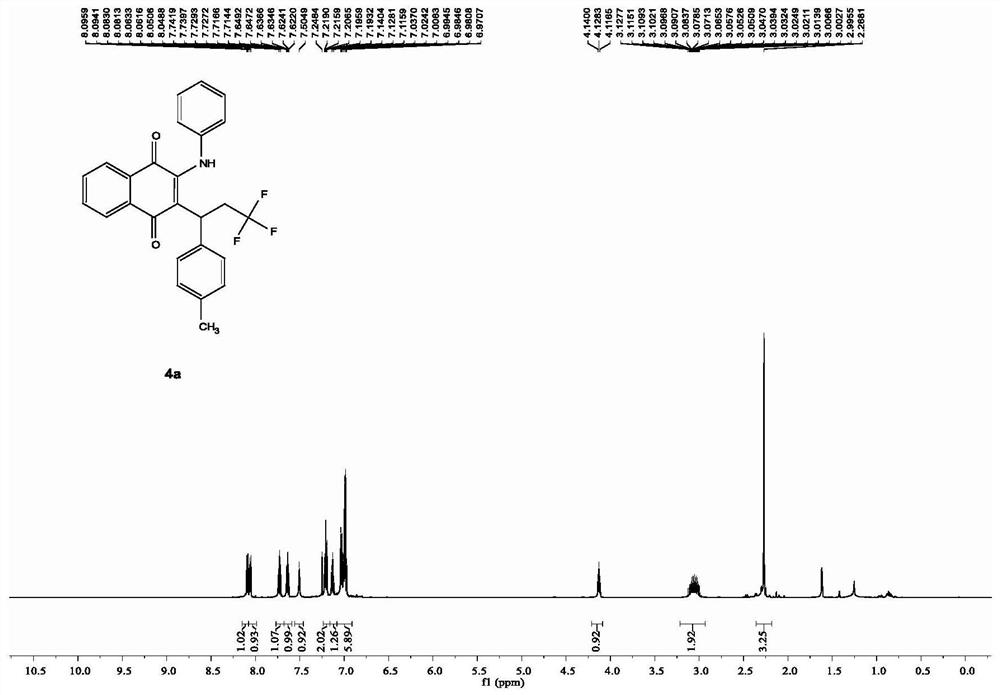
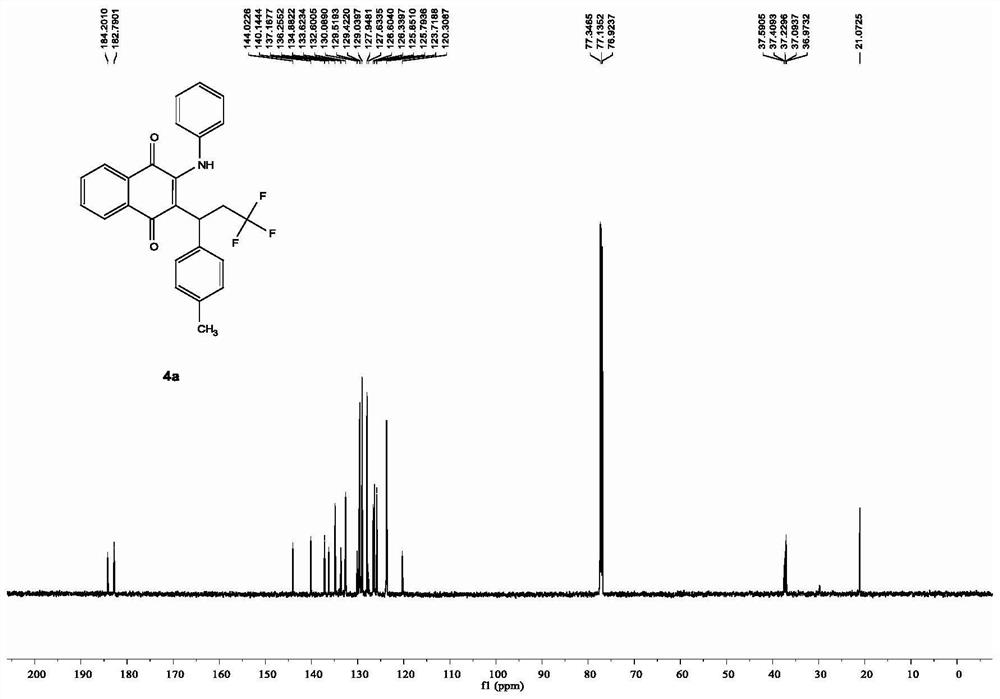
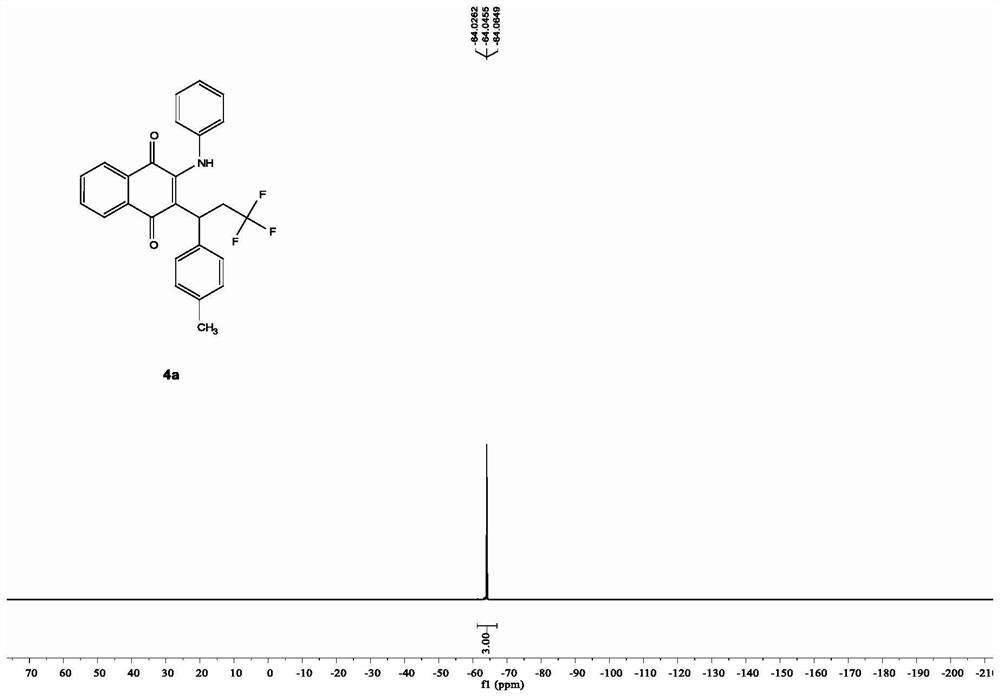
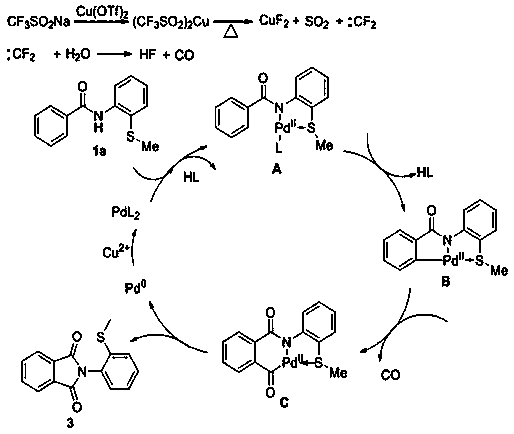
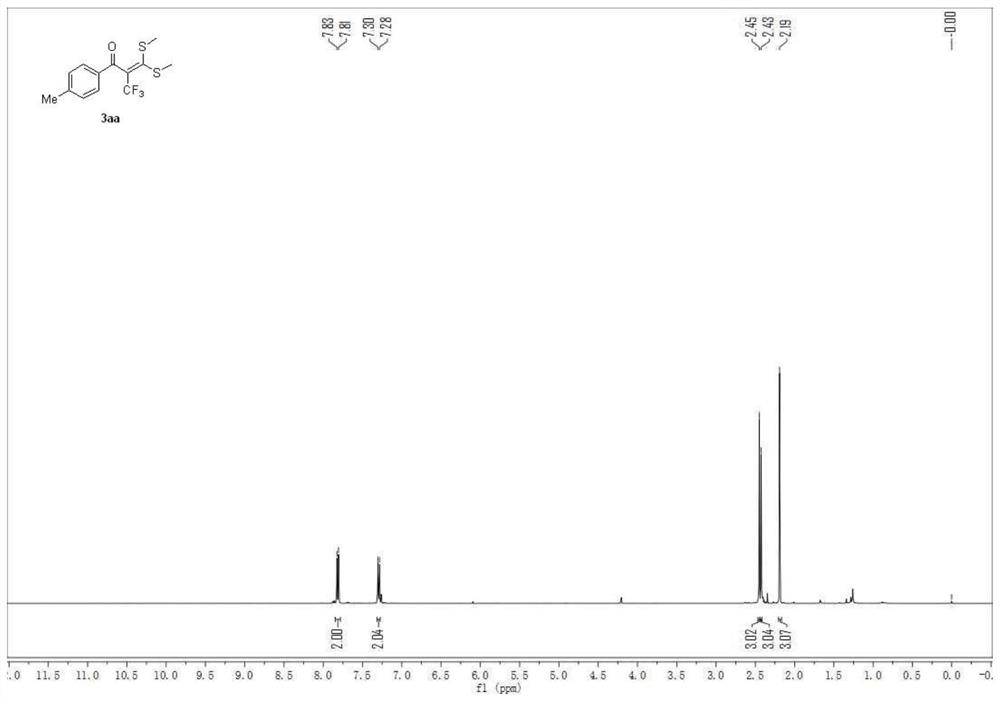
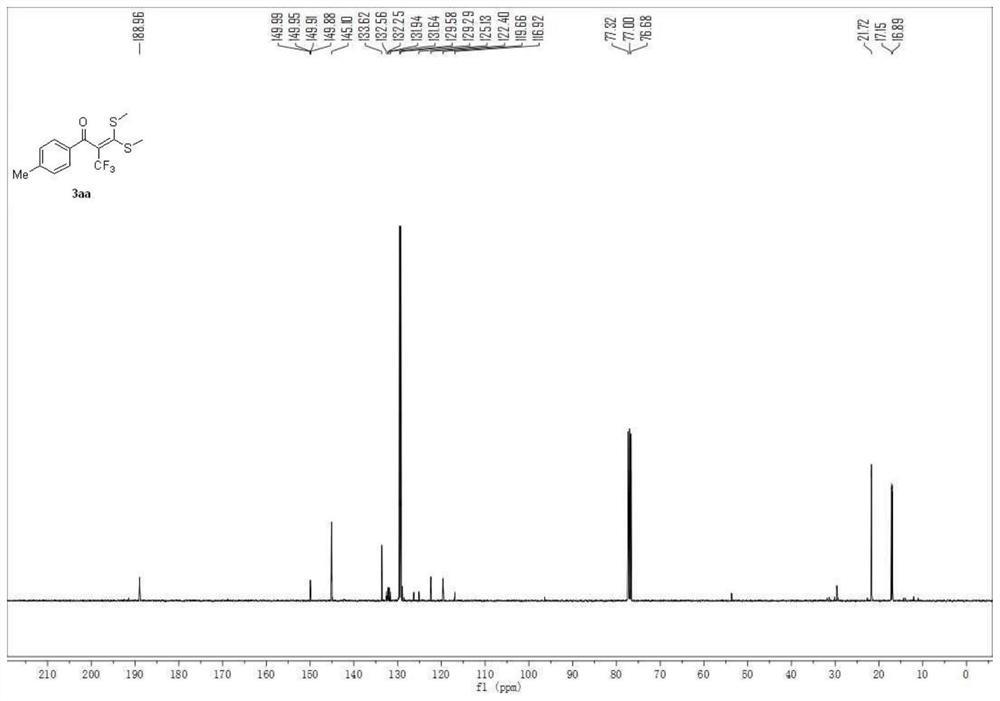
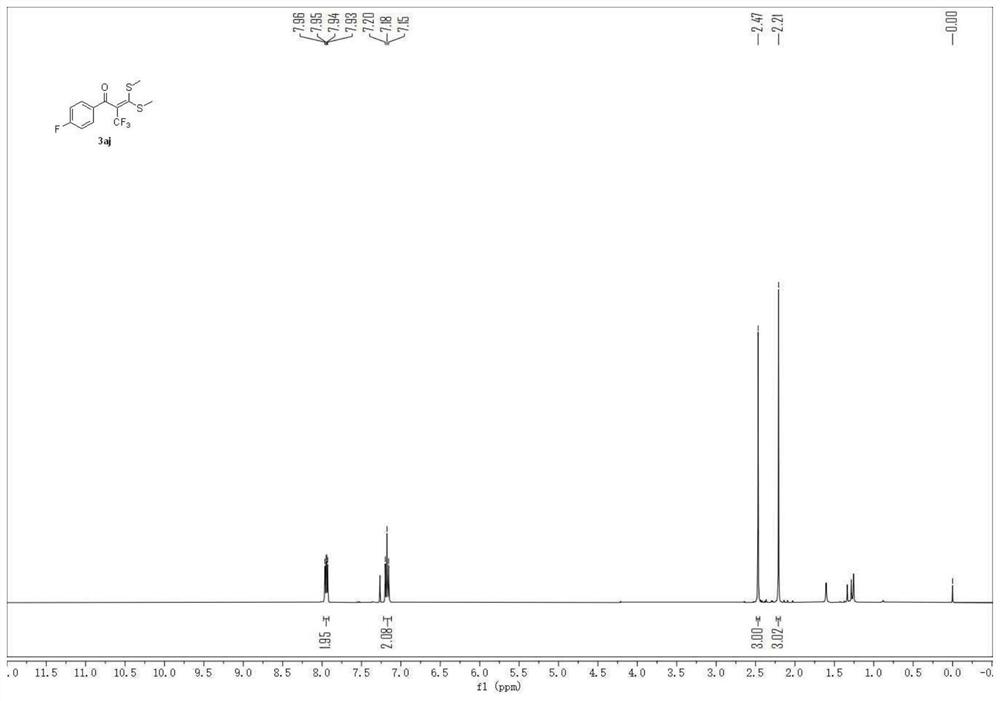
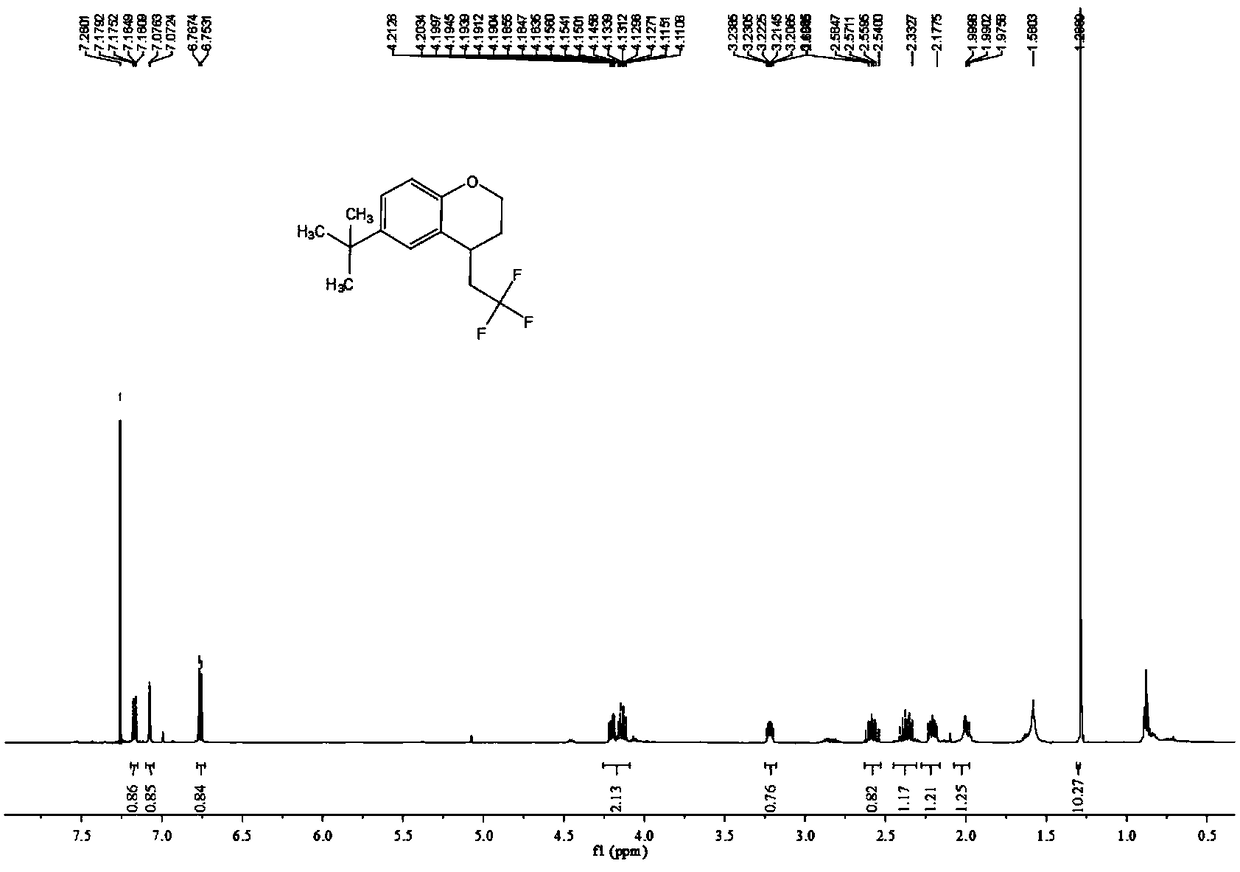
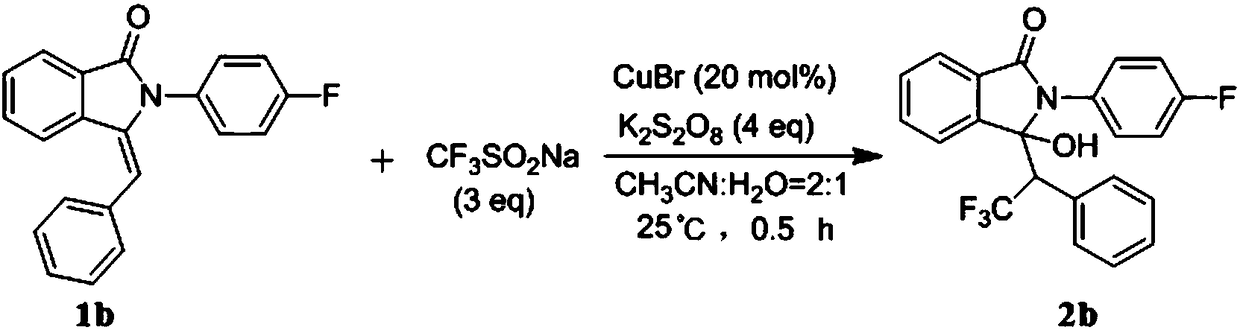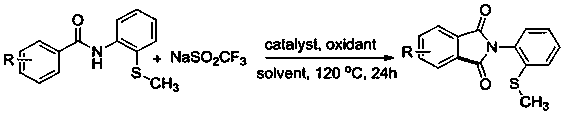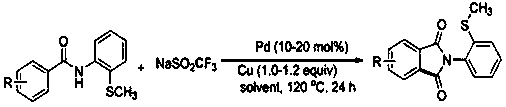Patents
Literature
50 results about "Sodium trifluoromethanesulfinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
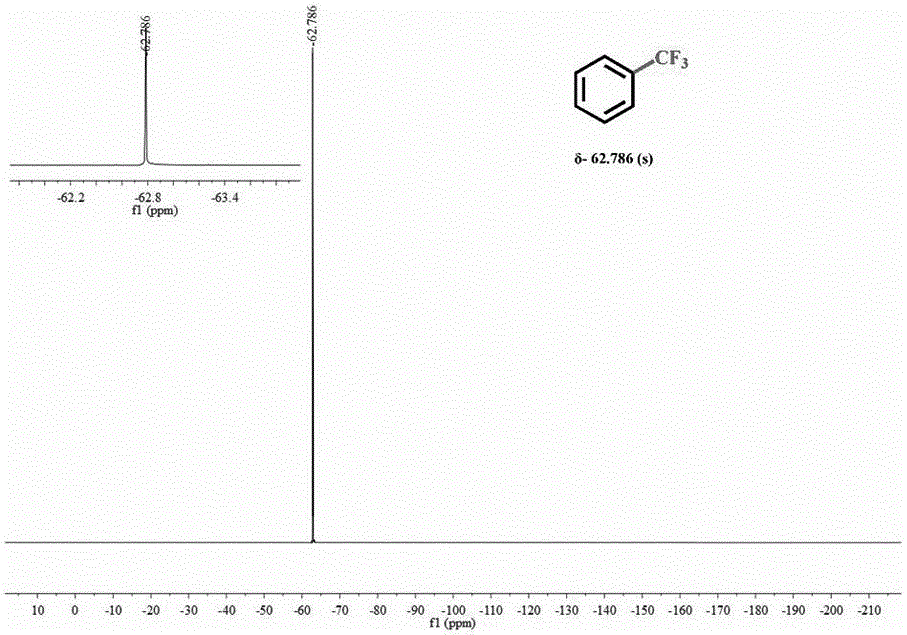
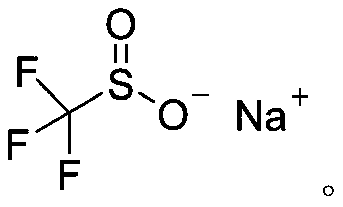
Sodium trifluoromethanesulfinate (CF₃SO₂Na) is the sodium salt of trifluoromethanesulfinic acid. Together with t-butyl hydroperoxide, an oxidant, this compound was found to be a suitable reagent for introducing trifluoromethyl groups onto electron-rich aromatic compounds by Langlois; this reagent is also known as the Langlois reagent. This reaction operates via a free radical mechanism.
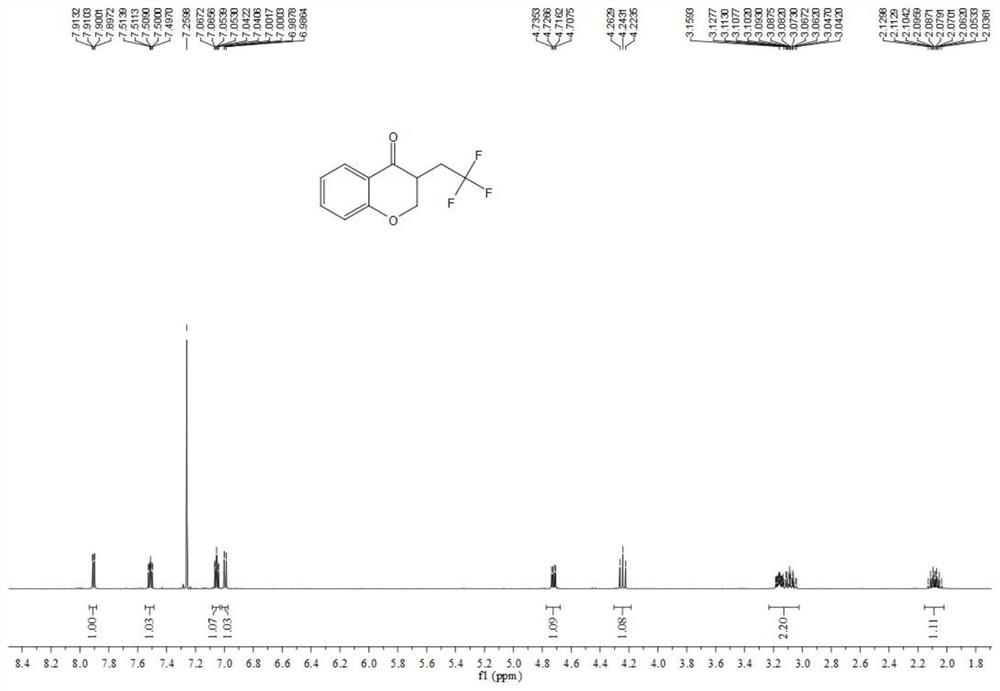
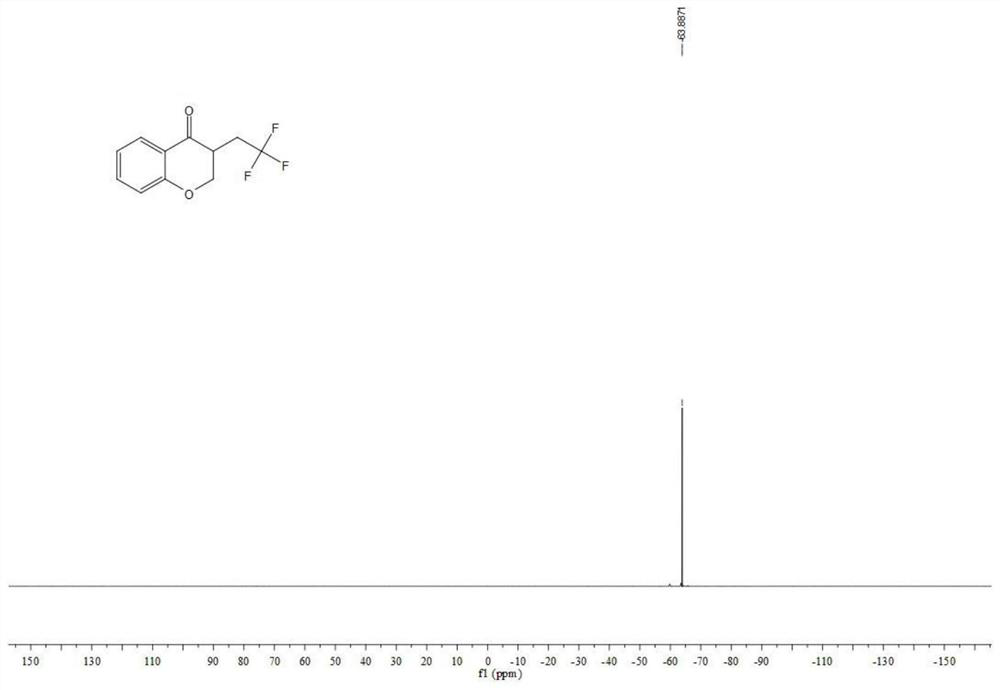
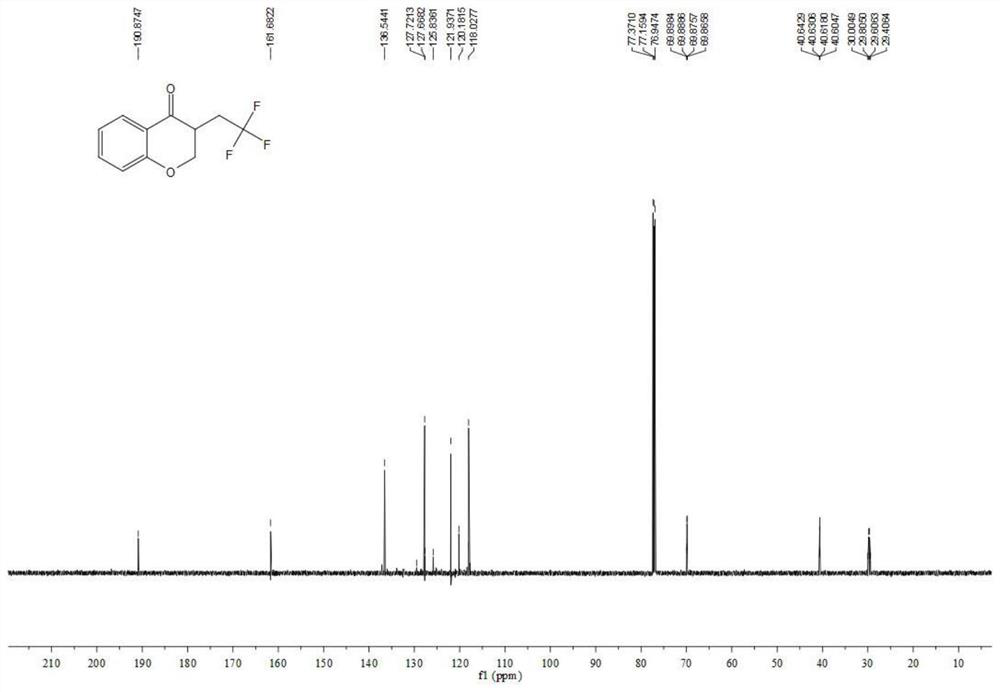
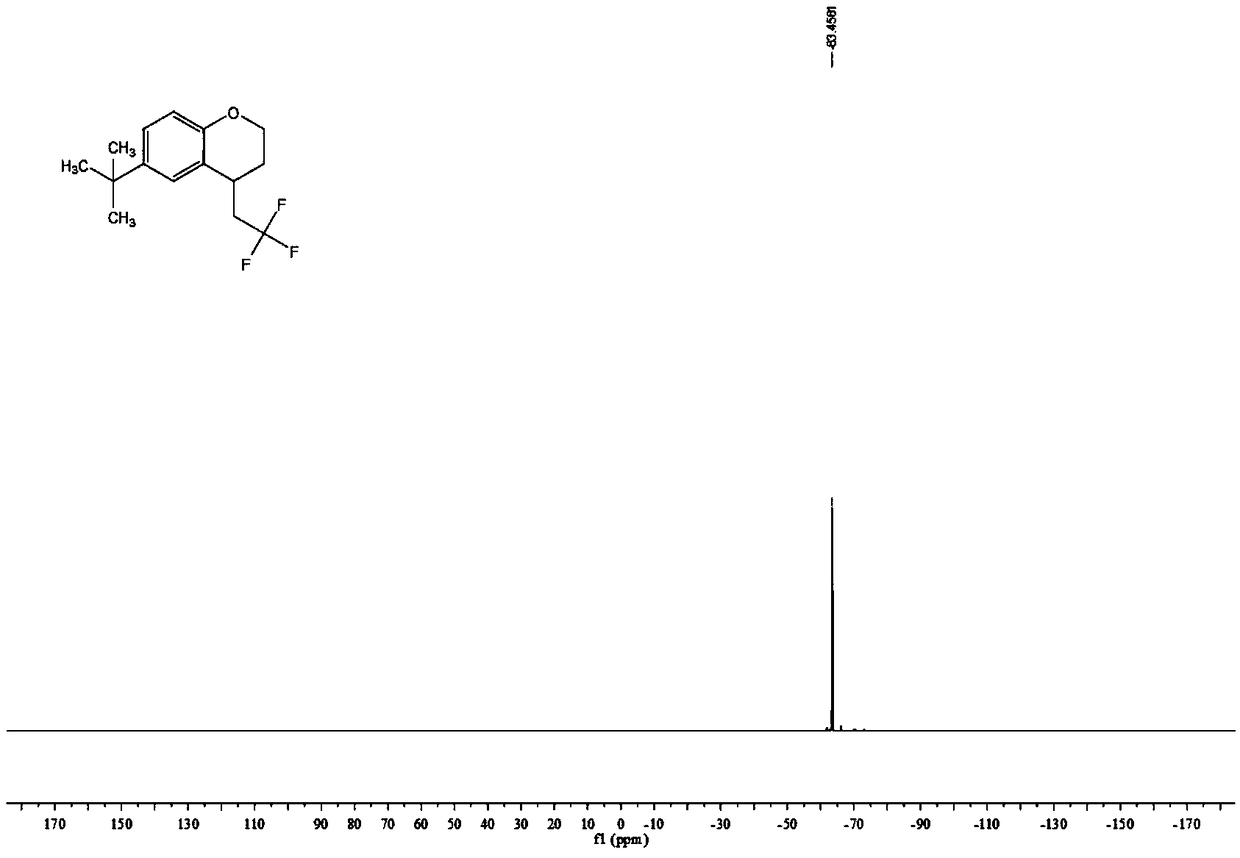
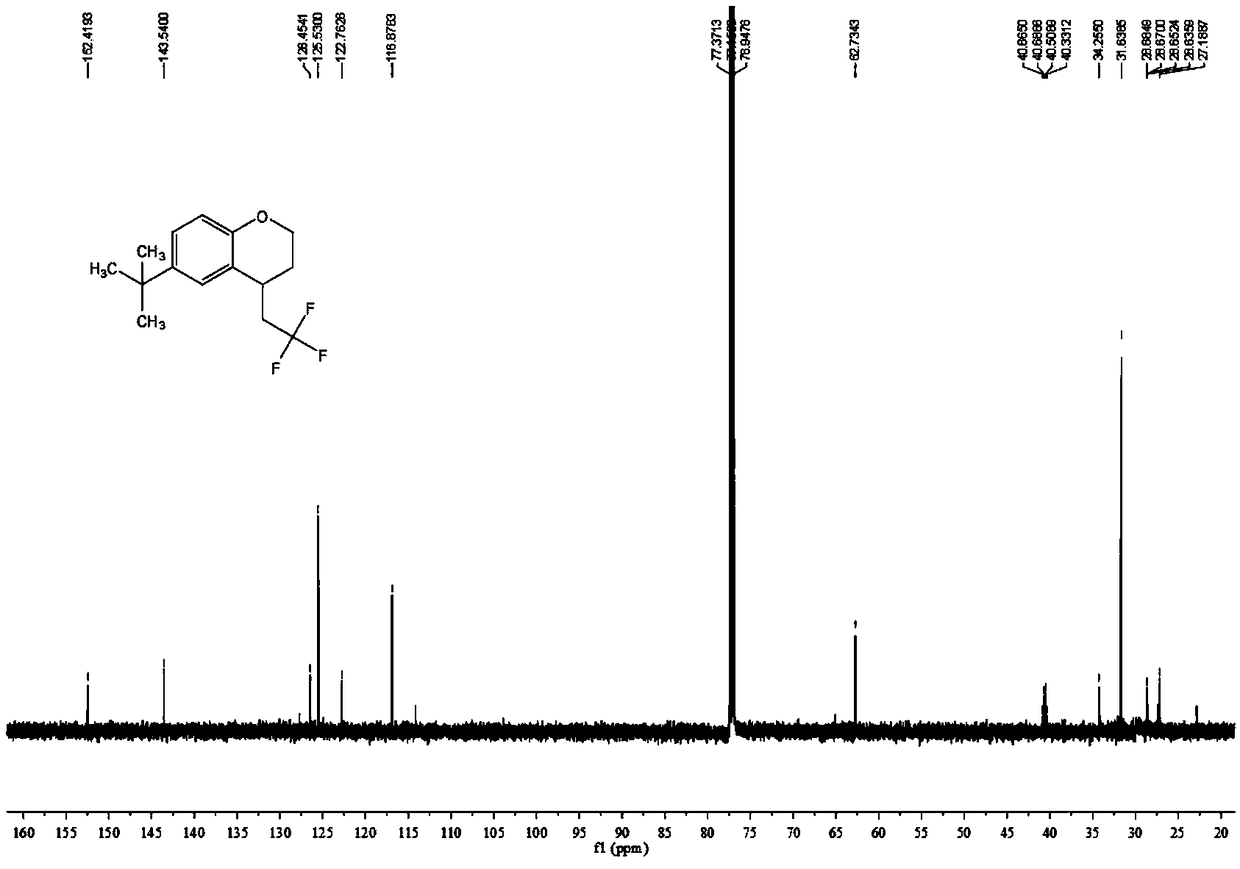
Chromane compound and preparation method thereof
InactiveCN109320489AMild reaction conditionsEasy to operateOrganic chemistryTrifluoromethylationSolvent
The invention discloses a chromane compound and a preparation method thereof. The preparation method comprises the following specific steps: dispersing olefin shown as a structure (I), a trifluoromethyl reagent shown as a structure (II) and an oxidizing agent in a solvent; heating and stirring the mixture to obtain the chromane compound shown as a structure (III), wherein the structure (III) is shown in the description. The invention further provides a novel method for building a trifluoromethylation chromane compound by taking an olefin compound (I) as a starting raw material of a reaction and sodium trifluoromethanesulfonate (II) as a trifluoromethyl source, and persulfate as an oxidizing agent, and performing free radical addition, free radical arylation cyclization and oxidization on trifluoromethyl and the olefin. The method has the advantages of mild reaction conditions, easiness in operation, diverse products, and the capability of realizing scale production.
Owner:XINYANG NORMAL UNIVERSITY
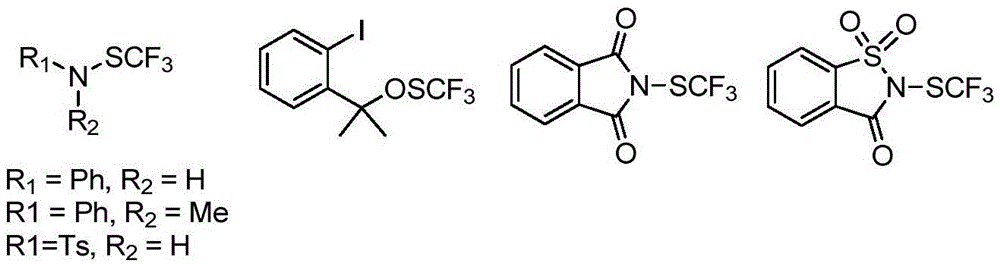
Trifluoromethylthiolation reagent and application thereof
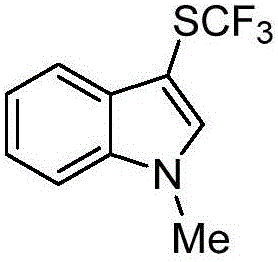
The invention discloses a trifluoromethylthiolation reagent and the application of the trifluoromethylthiolation reagent. The application of the trifluoromethylthiolation reagent comprises the following steps: adding sodium trifluoromethanesulfinate, indole and derivatives of the indole, appropriate amounts of dimethyl phosphate, iodine elements and copper acetate in a reactor containing a toluene solvent, stirring for 12h under the protection of nitrogen at the temperature of 110 DEG C; filtering reaction produced liquid, and distilling off low-boiling point substances; performing ethyl acetate / petroleum ether column chromatography isolation to obtain the product, 3-trifluomethylthio indole. The trifluoromethylthiolation reagent, namely sodium trifluoromethanesulfinate which is low in cost and readily available, is used, so that the synthesizing cost is greatly reduced and the process is simple to operate.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of alpha-trifluoromethylthio substituted acetophenone compound
ActiveCN108569942AHigh yieldEasy to prepareMercapto/sulfide group formation/introductionSulfide preparationAcetophenoneEthane Dichloride
The invention discloses a preparation method of an alpha-trifluoromethylthio substituted acetophenone compound. The preparation method comprises the following steps: by taking aryl ketone as a substrate, sodium trifluoromethanesulfinate as a trifluoromethylthio reagent, triphosgene as an additive, pyridine as a catalyst and dichloroethane as a solvent, in the nitrogen protection mode, stirring for12 hours at the temperature of 60 DEG C, performing a TLC tracking reaction, and conducting column chromatography isolation after sufficiently completing the reaction, thereby obtaining the alpha-trifluoromethylthio substituted acetophenone compound. According to the preparation method, the triphosgene is used as the reaction additive, so that using an expensive trifluoromethylthio reagent is avoided, but so far, a method for introducing trifluomethylthio has not been reported, the preparation method is simple and convenient, is low in cost, and is high in yield, a product is directly obtained, without any transition metal catalysis, and therefore, the preparation method is extremely high in practical popularization value.
Owner:UPCHEM CHINA
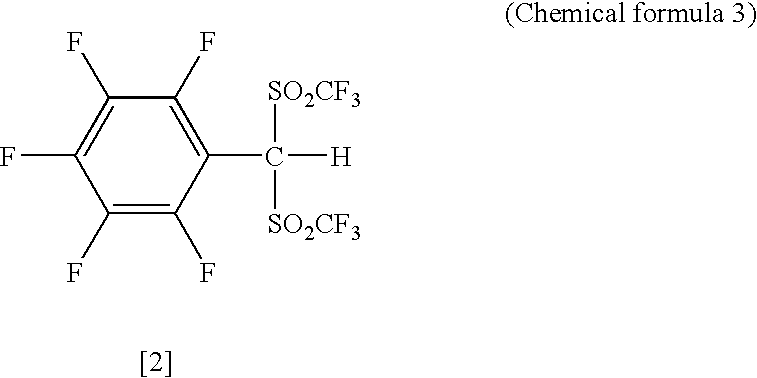
Arylbis (perfluoroalkylsulfonyl)methane and metallic salt thereof, and methods for producing the same
InactiveUS20050070741A1High yieldEasy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTrifluoromethanesulfonic anhydrideLithium
The present invention provides a method for producing various types of arylbis(perfluoroalkylsulfonyl)methane having a bulky aryl group and an electron-accepting aryl group in which synthesis was conventionally considered to be difficult, at high efficiency; a novel arylbis(perfluoroalkylsulfonyl)methane that can be widely applied to asymmertric catalyst, various types of functional materials and the like; and a metallic salt thereof. In addition, excellent catalysts are also provided. An aryl halomethane is reacted with a sodium trifluoromethane sulfinate, the arylmethyl triflone produced thereby is reacted with a t-BuLi and the like, the lithium salt of the arylmethyl triflone obtained is reacted with a trifluoromethane sulfonic acid anhydride, and an arylbis (trifluoromethylsulfony)methane such as pentafluorophenylbis(triflyl)methane, {4-(pentafluorophenyl)-2,3,5,6-tetrafluorophenyl}bis(triflyl)methane and the like are obtained at a high yield.
Owner:JAPAN SCI & TECH CORP
Preparation method of ortho-trifluoromethyl-substituted azide compound
ActiveCN108640808AMild method conditionsEasy to handleSulfonic acid amide preparationFunctional group formation/introductionAzidotrimethylsilaneSolvent
The invention discloses a preparation method of an ortho-trifluoromethyl-substituted azide compound. The preparation method of the ortho-trifluoromethyl-substituted azide compound comprises the following steps of adding manganese salts, olefin derivatives, sodium trifluoromethanesulfinate, azidotrimethylsilane and a peroxy compound into a solvent in the presence of inert gas, and carrying out reaction at 25-75 DEG C for 6-12 hours; and then, carrying out column chromatography separation so as to be subjected to purifying, and obtaining the ortho-trifluoromethyl-substituted azide compound. Themole ratio of the manganese salts to the olefin derivatives to the sodium trifluoromethanesulfinate to the azidotrimethylsilane to the peroxy compound is 0.1-0.2) to 1 to (1.5-2.5) to (2.5-3.5) to (2.5-3.5); and the dosage of the solvent is 6-7 milliliters of the solvent for per millimole of the olefin derivatives. The preparation method of the ortho-trifluoromethyl-substituted azide compound is low in raw material costs, mild in reaction conditions, simple in post-treatment and suitable for industrial production.
Owner:HEBEI UNIV OF TECH
Quinone-catalyzed trifluoromethylation photocatalytic synthesis method
ActiveCN105503510AReduce pollutionEasy to makeOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTrifluoromethylationQuinone
The invention discloses a quinone-catalyzed trifluoromethylation photocatalytic synthesis method. According to the method, the using problem of highly-corrosive and highly-toxic fluorinating reagents such as hydrogen fluoride and sulfur tetrafluoride in an existing industrial trifluoromethylation reaction is solved, the heavy metal pollution problem of a catalyst in industry is avoided, and the good actual application prospect is achieved. The metal-free photocatalytic trifluoromethylation reaction is achieved by taking simplest and cheapest benzoquinone and derivatives thereof as a photocatalyst and taking a Langlois reagent-sodium trifluoromethanesulfinate (CF3SO2Na) as a trifluoromethyl source under the mild visible light condition, and a complete full-cycle reaction is achieved through a homemade fixed bed reactor. Trifluoromethyl modification can be further performed on organic molecules with the bioactivity and the pharmaceutical activity according to the actual needs. The method has the advantages that the synthesis condition is mild, the raw materials are cheap and easy to obtain, the environmental pollution is low, large-scale industrial production is facilitated, and the significant economic and social benefit is achieved.
Owner:FUZHOU UNIV
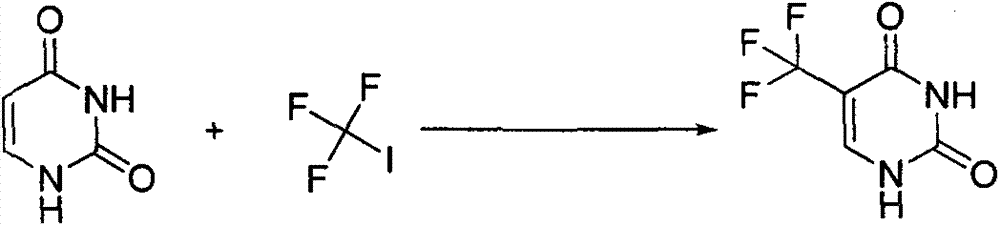
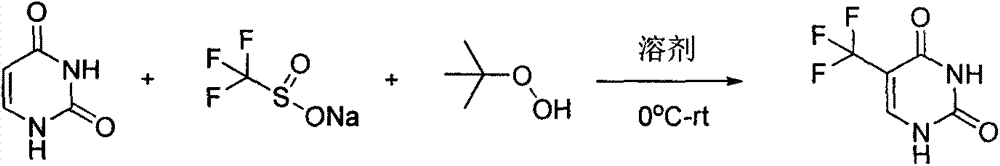
Synthesis method of 5-(trifluoromethyl) uracil
The invention discloses a synthesis method of 5-(trifluoromethyl) uracil. The method comprises the step of fluorinating uracil by use of sodium trifluoromethanesulfinate and tert-butyl hydrogen peroxide in the presence of an effective dose of catalyst, wherein the catalyst is copper sulfate and the weight of the catalyst accounts for 10-50% of that of the uracil; furthermore, by regulating the equivalent number of the sodium trifluoromethanesulfinate, the cost consumed by raw materials can be minimized, and the equivalent number is 1.5.
Owner:NANJING ANYUAN BIO PHARMA TECH CO LTD
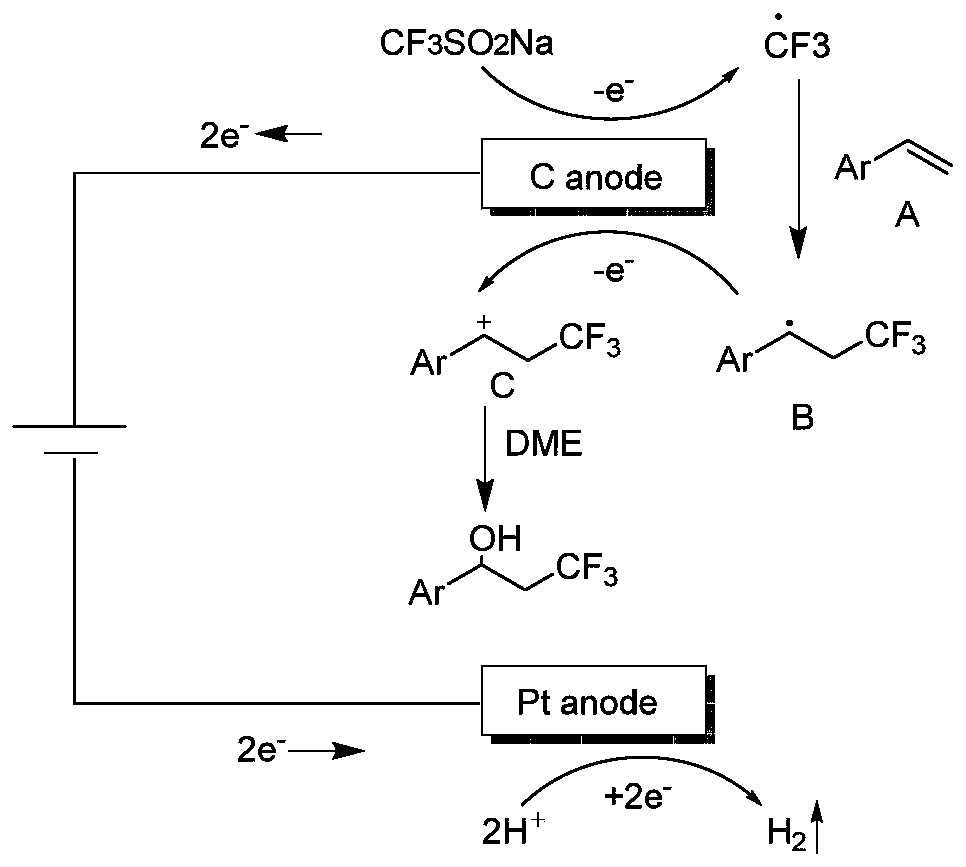
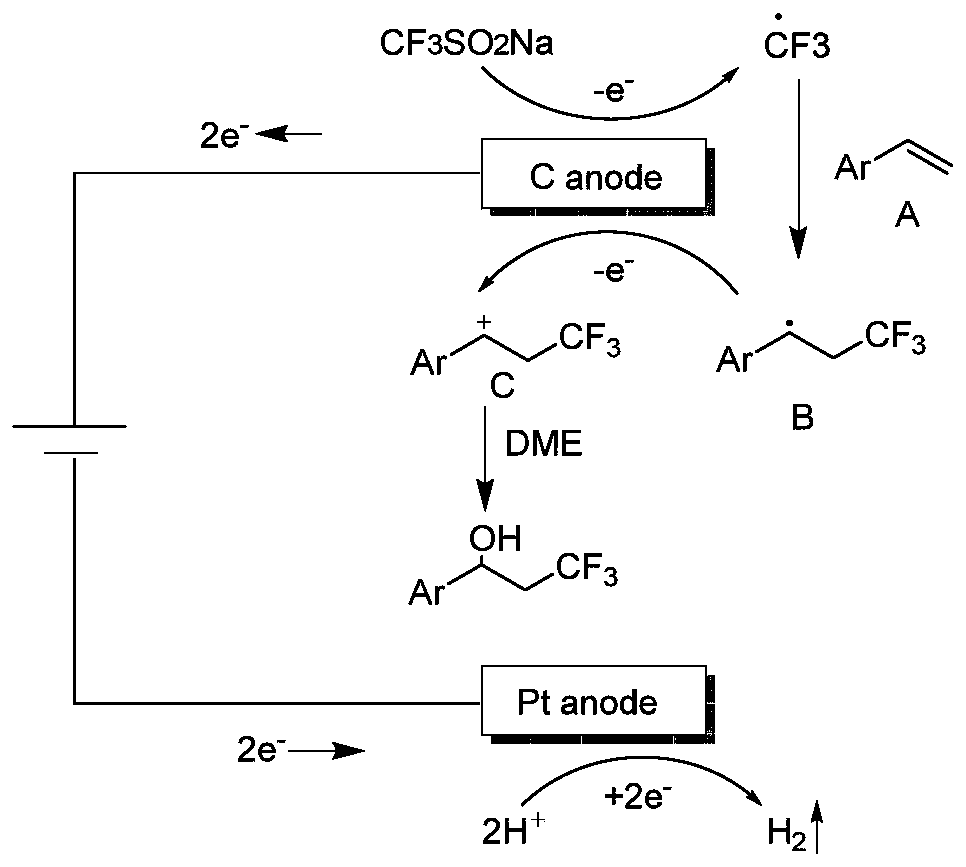
Synthesis method of beta-trifluoromethyl substituted alcohol organic molecule
ActiveCN111155142ASimple ingredientsReduce usageElectrolysis componentsElectrolytic organic productionFluoroacetic acidPtru catalyst
The invention discloses a synthesis method of a beta-trifluoromethyl substituted alcohol organic molecule. The method comprises the following steps: firstly, mixing a styrene substrate, sodium trifluoromethanesulfinate and an electrolyte salt lithium perchlorate; adding a solvent ethylene glycol dimethyl ether and a strong acid trifluoroacetic acid while stirring, then inserting a counter electrode below the liquid level of the solvent, and carrying out an electrocatalytic reaction under the conditions of constant current of 15 + / -3 mA and stirring to obtain the beta-trifluoromethyl substituted alcohol organic molecule corresponding to the substrate. The beta-trifluoromethyl substituted alcohol organic molecule is synthesized by using an electro-catalysis means, and an active intermediateis generated through direct initiation of single electron transfer, so the use of a metal catalyst and a peroxidant is avoided, the reaction system is green and efficient, and a practical and effective path is provided for the synthesis of the beta-trifluoromethyl substituted alcohol organic molecule.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol
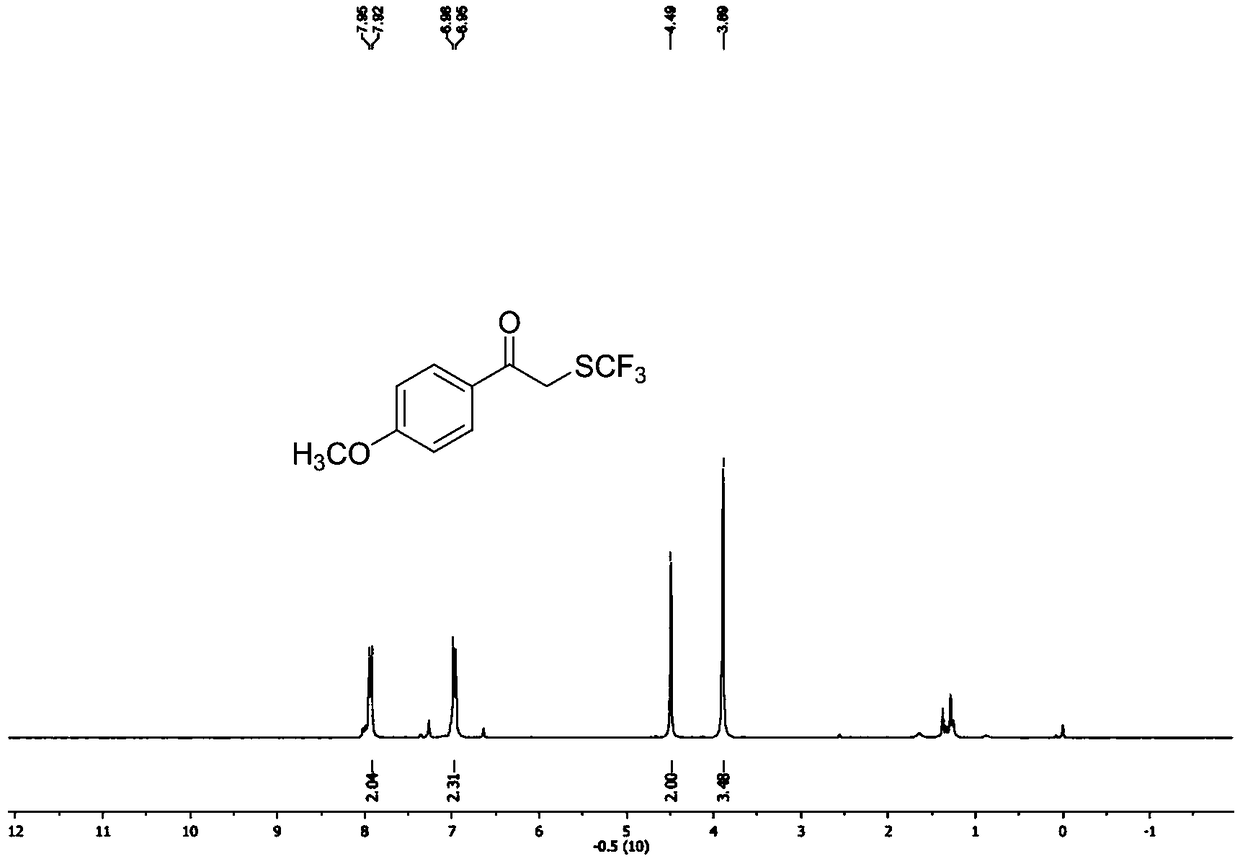
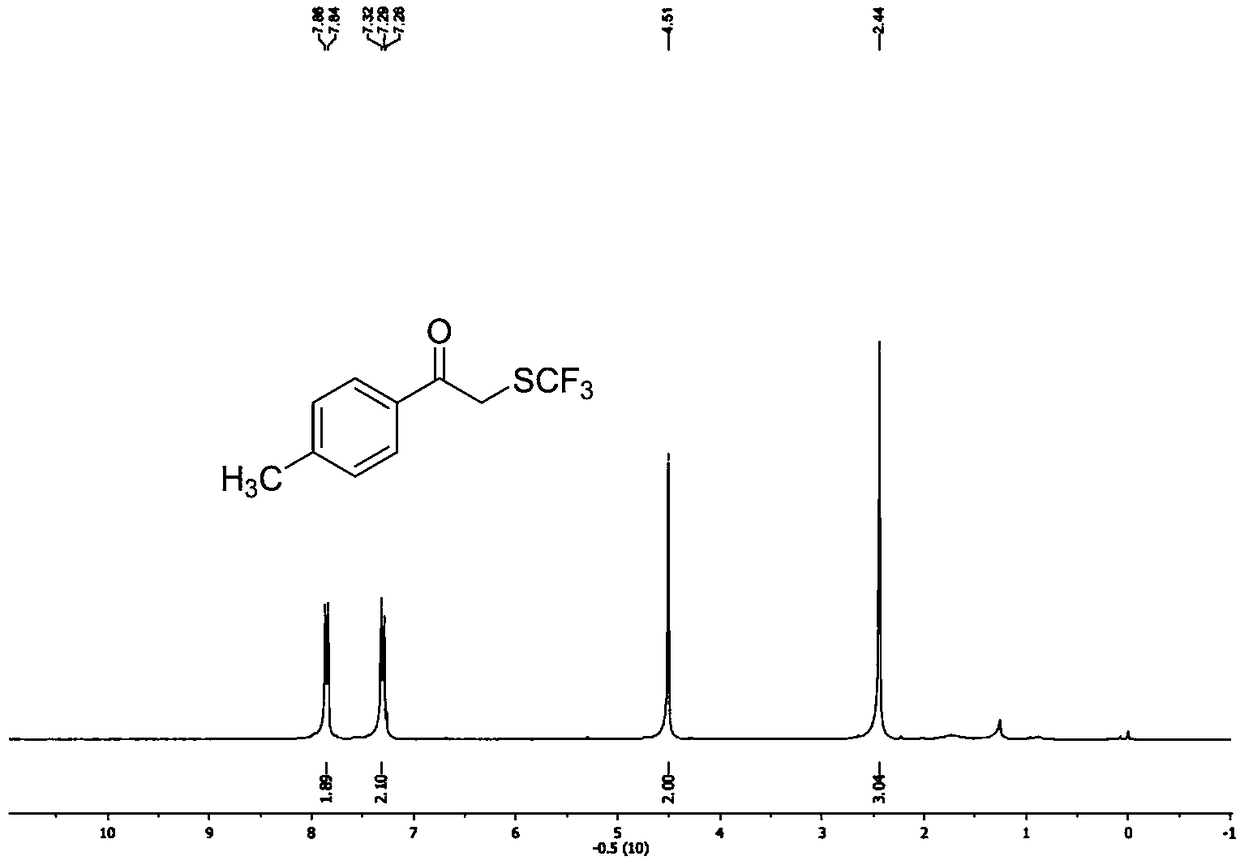
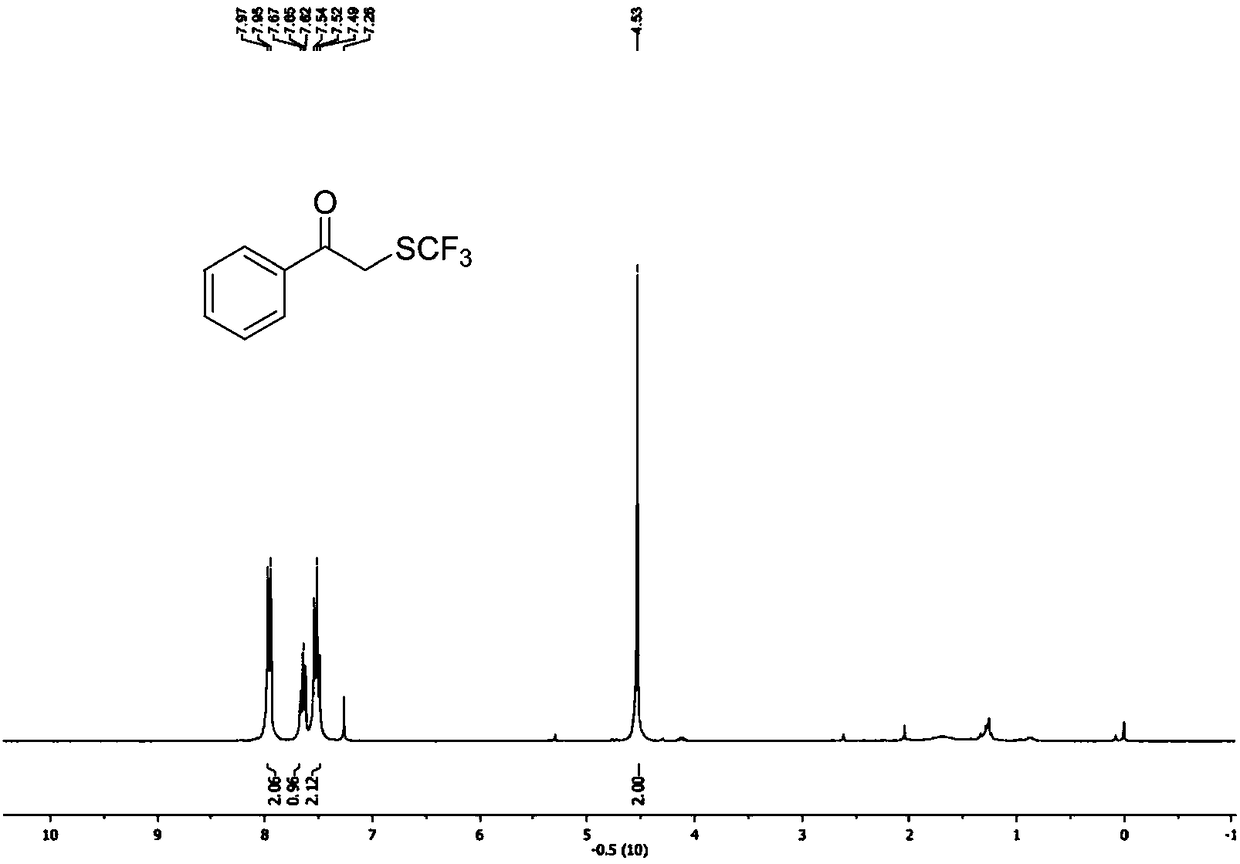
ActiveCN111205185AWide range of sources of light energyLow costOrganic compound preparationCarboxylic acid esters preparationAlcoholOrganosolv
The invention relates to a preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol. The method comprises the following steps: by using visible light as an energy source, aliphaticolefin and sodium trifluoromethanesulfinate as reaction substrates, organic manganese salt as a catalyst and oxygen-containing atmosphere as an oxidant, performing reacting in an organic solvent at room temperature to obtain [beta]-trifluoromethyl alcohol. The method is greener and safer, is suitable for various substituted aliphatic olefins, and is mild and efficient in reaction.
Owner:SUZHOU UNIV
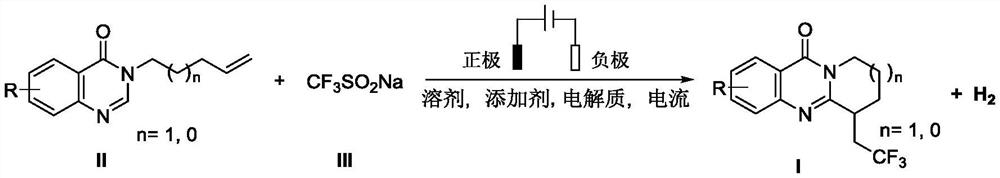
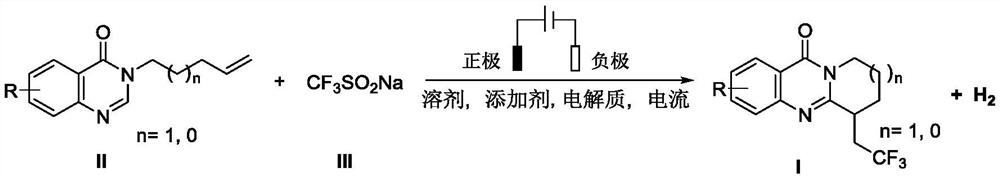
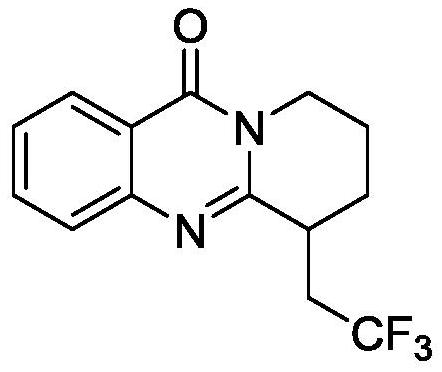
Electrochemical synthesis method of trifluoromethyl-substituted polycyclic quinazolinone derivative
PendingCN114108013ARaw materials are easy to getEasy to operateElectrolytic organic productionElectrodesPtru catalystCatalytic oxidation
The invention relates to the technical field of electrochemical synthesis, in particular to an electrochemical synthesis method of a trifluoromethyl-substituted polycyclic quinazolinone derivative. The method comprises the following steps: sequentially adding 3-alkene alkyl substituted quinazolinone, sodium trifluoromethanesulfinate, an electrolyte, an additive and a solvent into an unseparated electrolytic cell serving as a reactor, respectively inserting a positive electrode and a negative electrode of an electrode into a reaction solution, switching on current, keeping constant current, stirring and reacting, and after the reaction is finished, performing column chromatography separation to obtain the 3-alkene alkyl substituted quinazolinone. The trifluoromethyl substituted polycyclic quinazolinone derivative is obtained. According to the method, a metal catalyst and an oxidizing agent are not needed, only electro-catalytic oxidation is used, a byproduct is hydrogen, and the method has the characteristics that raw materials are easy to obtain, operation is simple, electrolyte and a solvent can be used mechanically and the like, and has good popularization and implementation value and social and economic benefits.
Owner:江苏信和生物医药有限公司
Application of biomass-supported copper-catalyzed three-component reaction in preparation of fluorine-containing medicine
ActiveCN113444076AEfficient cycle capacityHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsQuinoxalinePtru catalyst
The invention relates to an application of a biomass-supported copper-catalyzed three-component reaction in preparation of a fluorine-containing medicine, which comprises the following steps: catalyzing quinoxalinone and derivatives thereof, indole and derivatives thereof and sodium trifluoromethanesulfinate by using a copper catalyst taking chitosan as a carrier, so as to synthesize the 3-(2-(trifluoromethyl)-indol-3-yl) quinoxalin-2-one derivative from the three components by a one-pot method. The heterogeneous biomass copper-loaded catalyst is adopted to catalyze the reaction, and the problems that a traditional homogeneous catalyst cannot be recycled, and metal residues exist are solved; and the 3-(2-(trifluoromethyl)-indol-3-yl)quinoxalin-2-one compound with different substituent groups is expanded, the biomass supported copper catalyst can be cyclically catalyzed for multiple times, the target product keeps good yield, and it is indicated that the catalyst has efficient cycle capacity and can promote the possibility of industrialization of a reaction system.
Owner:ZHEJIANG SHUREN UNIV
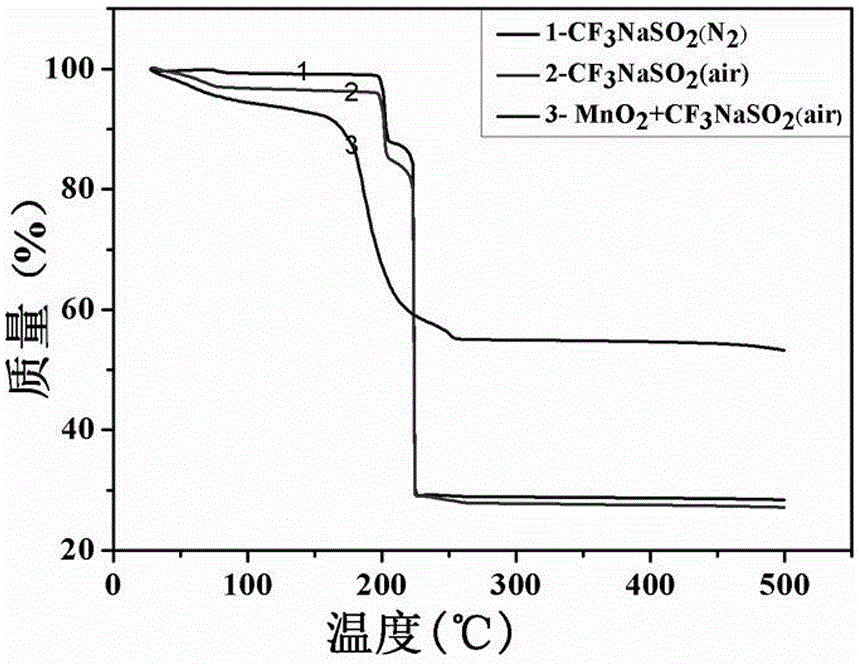
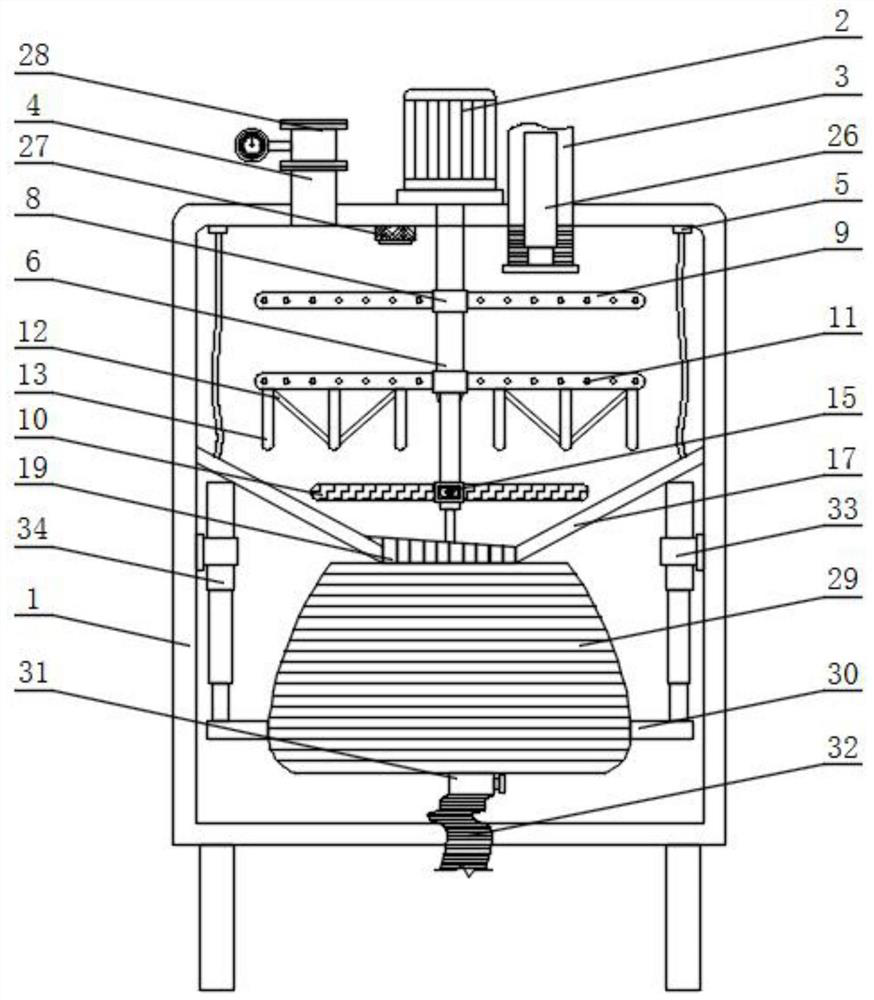
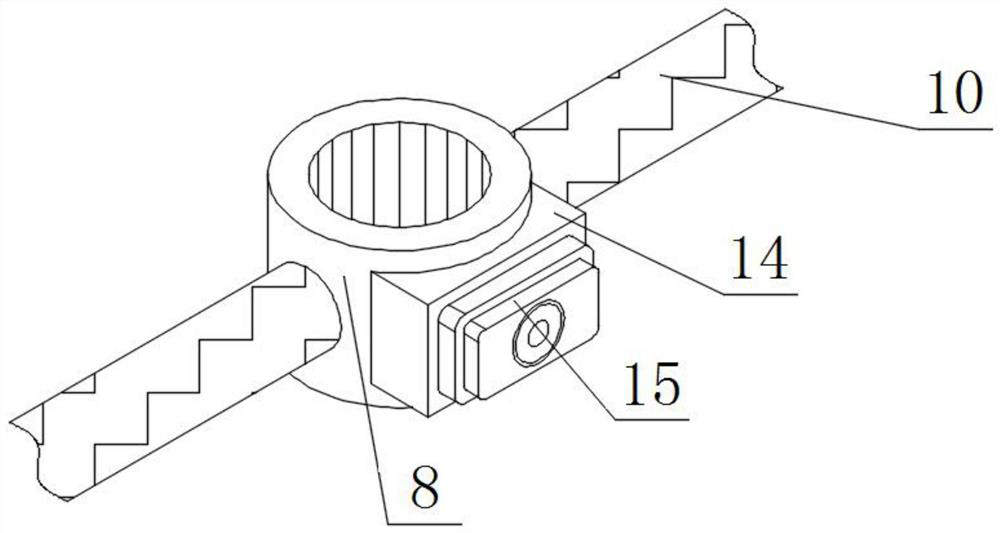
High-content sodium trifluoromethanesulfinate refining equipment and high-content sodium trifluoromethanesulfinate manufacturing method
InactiveCN113976014AIncrease temperatureImprove solubilityOrganic chemistryRotary stirring mixersMechanical engineeringIndustrial engineering
The invention discloses high-content sodium trifluoromethanesulfinate refining equipment, which comprises an equipment box, a servo motor, an air suction pipe, feeding pipes and fixing blocks, wherein the servo motor is mounted at the top of the equipment box, the air suction pipe is mounted at the top of the equipment box, a plurality of sets of feeding pipes arranged front and back are installed at the top of the servo motor, four sets of fixing blocks arranged in a square shape are installed on the top wall of the equipment box, the output end of the servo motor is provided with a rotating cylinder through a shaft, the inner wall of the rotating cylinder is provided with a movable cylinder, the outer surface of the rotating cylinder and the outer surface of the movable cylinder are both provided with sleeve rings, and the outer surfaces of two sets of sleeve rings are both provided with two sets of stirring rods which are symmetrically arranged. According to the invention, the volume of a solution entering an equipment box can be monitored in real time, then the additive dissolving speed can be increased in a stirring and heating mode, and the purity of sodium trifluoromethanesulfinate can be improved by adopting the preparation method provided by the invention.
Owner:江苏优普生物化学科技股份有限公司
Synthesis process of high-quality sodium trifluoromethanesulfinate
InactiveCN112457227AEasy to operateHigh purityOrganic chemistrySodium dithioniteIndustrial engineering
The invention discloses a synthesis process of high-quality sodium trifluoromethanesulfinate. The synthesis process comprises the following steps: A, mixing sodium hydrosulfite with trifluoromethyl bromide and sodium hydroxide, adding the mixture into a reaction kettle, and heating to react to obtain a mixed solution of sodium trifluoromethanesulfinate and sodium bromide; B, feeding the mixed solution obtained in the step A into a layering kettle, and standing for layering to obtain a supernatant and a lower filtrate; C, extracting supernatant, adding the lower filtrate into a stirring kettle,and adding an extracting agent into the stirring kettle for extraction reaction under stirring; D, feeding the reaction product in the processing step C into a desolventizing kettle for desolventizing to obtain a semi-finished product; and E, finally, drying the semi-finished product through a high-speed centrifugal spray dryer to obtain sodium trifluoromethanesulfinate. The synthesis process issimple in operation, pollution-free in synthesis process, high in purity of sodium trifluoromethanesulfinate, high in yield, capable of effectively reducing production cost, capable of saving energy and reducing consumption and good in economic benefit.
Owner:江苏优普生物化学科技股份有限公司
Arylbis (perfluoroalkylsulfonyl) methane, metal salf of same, and processes for producing these
InactiveCN1481357AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsArylTrifluoromethanesulfonic anhydride
The present invention provides a method for producing various types of arylbis(perfluoroalkylsulfonyl)methane having a bulky aryl group and an electron-accepting aryl group in which synthesis was conventionally considered to be difficult, at high efficiency; a novel arylbis(perfluoroalkylsulfonyl)methane that can be widely applied to asymmertric catalyst, various types of functional materials and the like; and a metallic salt thereof. In addition, excellent catalysts are also provided. An aryl halomethane is reacted with a sodium trifluoromethane sulfinate, the arylmethyl triflone produced thereby is reacted with a t-BuLi and the like, the lithium salt of the arylmethyl triflone obtained is reacted with a trifluoromethane sulfonic acid anhydride, and an arylbis(trifluoromethylsulfony)methane such as pentafluorophenylbis(triflyl)methane, {4-(pentafluorophenyl)-2,3,5,6-tetrafluorophenyl}bis(triflyl)methane and the like are obtained at a high yield.
Owner:JAPAN SCI & TECH CORP
Synthesis method of bis [(trifluoromethyl) sulfonyl] methane
PendingCN111349030AHigh yieldHigh purityOrganic chemistryOrganic compound preparationElectrolytic agentAcyl group
The invention discloses a synthesis method of bis [(trifluoromethyl) sulfonyl] methane. The invention belongs to the technical field of battery electrolyte. The method comprises the following steps: adding sodium trifluoromethanesulfinate, methyl magnesium bromide and tetrabutylammonium iodide into an organic solvent to obtain a mixed solution; under the stirring condition, subjecting the mixed solution to heating reflux reaction for 8 to 12h; and cooling the reaction solution to room temperature, filtering, adding diethyl ether, adding tert-butyl lithium and trifluoromethanesulfonic anhydrideat -20 to -40 DEG C, stirring for 20-30 minutes, heating to 30-40 DEG C, continuing stirring for 20-30 minutes, washing, drying, filtering, and concentrating to obtain bis [(trifluoromethyl) sulfonyl] methane. The synthesis method is simple, and the obtained bis [(trifluoromethyl) sulfonyl] methane is high in yield, high in purity and low in moisture content.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
Method using manganese dioxide to catalyze trifluoromethylation of arenes or heterocyclic arenes
InactiveCN106831317ALow costMild reaction conditionsHalogenated hydrocarbon preparationTrifluoromethylationAcetonitrile
The invention discloses a method using manganese dioxide to catalyze trifluoromethylation of arenes or heterocyclic arenes. According to the method, the manganese dioxide serves as a catalyst, sodium trifluoromethanesulfinate serves as a source of trifluoromethyl groups, acetonitrile serves as a solvent, the trifluoromethylation of the arenes or the heterocyclic arenes is conducted at the low-temperature atmospheric condition. According to the method, the materials are cheap and easy to obtain, the reaction condition is mild, environmental pollution is low, products are easy to separate, the situation is avoided that highly-corrosive and highly-toxic fluorinated reagents are used in current industrial trifluoromethylation reactions, and the method has potential industrial application value.
Owner:FUZHOU UNIV
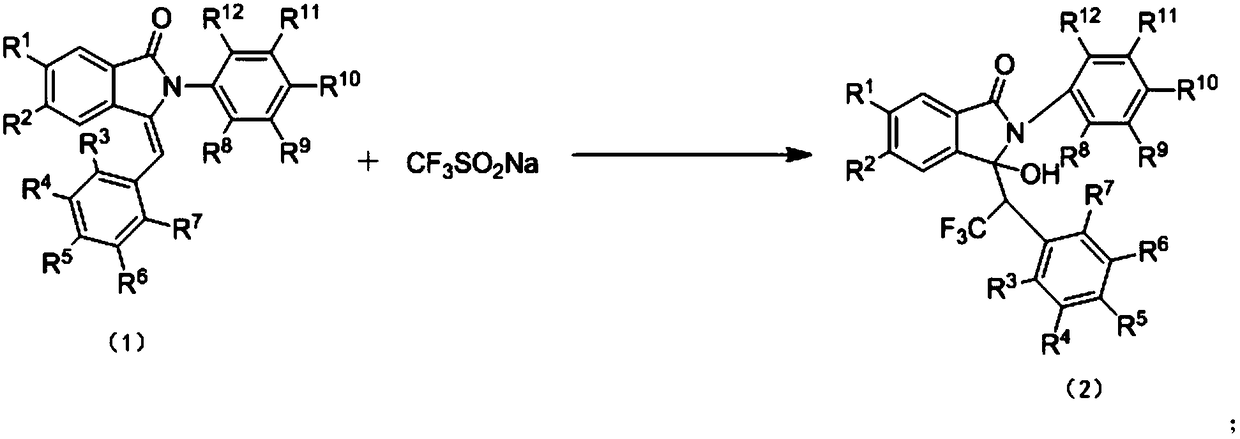
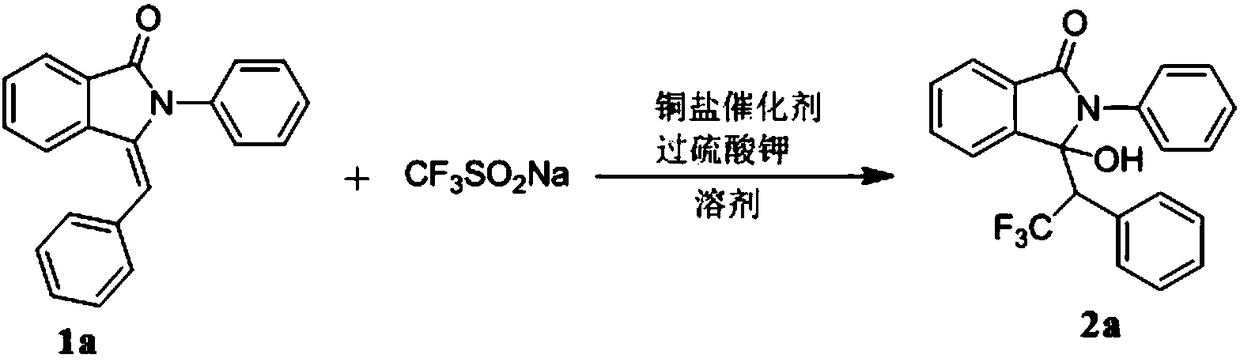
Preparation method of trifluoromethyl hydroxylated derivatives of isoindolinone
ActiveCN108440384ARaw materials are easy to getMild reaction conditionsOrganic chemistryHydrogenSolvent
The invention relates to a preparation method of trifluoromethyl hydroxylated derivatives of isoindolinone. The method comprises steps as follows: a substituted N-phenyl-3-benzalisoindole-1-one derivative shown as the formula (1) and sodium trifluoromethanesulfinate are subjected to a reaction in a solvent at 10-80 DEG C under the catalytic action of copper salt and oxidizing action of an oxidizing agent, the trifluoromethyl hydroxylated derivatives of isoindolinone shown as the formula (2) are obtained, and the reaction route is shown in the description, wherein R<1>, R<2>, R<3>, R<4>, R<5>,R<6>, R<7>, R<8>, R<9>, R<10>, R<11> and R<12> are independently selected from hydrogen, alkyl, alkoxy or halogen. With adoption of the method, multiple trifluoromethyl hydroxylated derivatives of N-phenyl-3-benzalisoindole-1-one can be obtained with high yield; reaction conditions are mild, operation and aftertreatment processes are simple, and the method is suitable for large-scale production.
Owner:SUZHOU UNIV
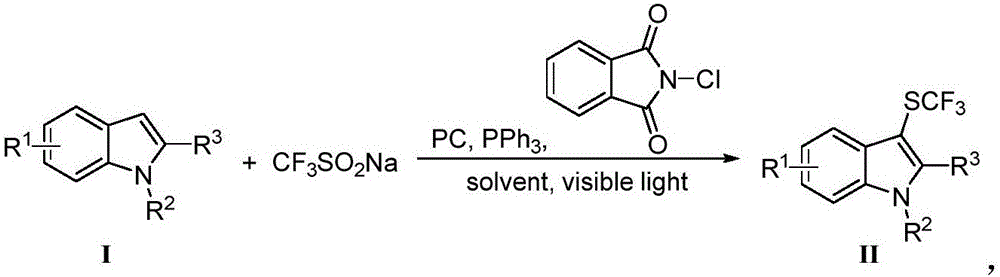
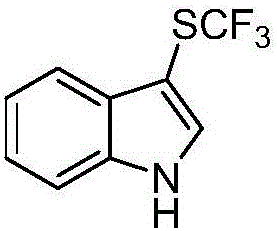
Method for preparing trifluoro-methylmercapto-substituted indole compound
InactiveCN106748608AChemically stableLow toxicityMercapto/sulfide group formation/introductionSulfonateEosin Y
The invention discloses a method for preparing a trifluoro-methylmercapto-substituted indole compound. The method comprises the following step: by taking trifluoro-methyl sodium sulfonate as a trifluoro-methylmercapto source and triphenylphosphine as a reducing agent, under the action of a visible light catalyst eosin Y, and enabling a substituted indole compound, the trifluoro-methyl sodium sulfonate and triphenylphosphine to react with N-chloro-o-phthalimide, thereby obtaining the trifluoro-methylmercapto-substituted indole compound. The method disclosed by the invention is simple, a trifluoro-methylmercapto reagent used in the method is low in toxicity, and no heating is needed in the preparation process, so that the energy can be saved, the environment can be protected, and direct trifluoro-methylmercapto reaction of a 3-position C (sp2)-H bond of an indole compound is achieved.
Owner:NANJING UNIV OF SCI & TECH
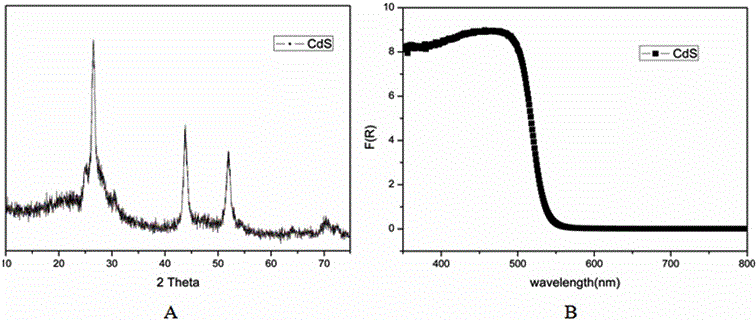
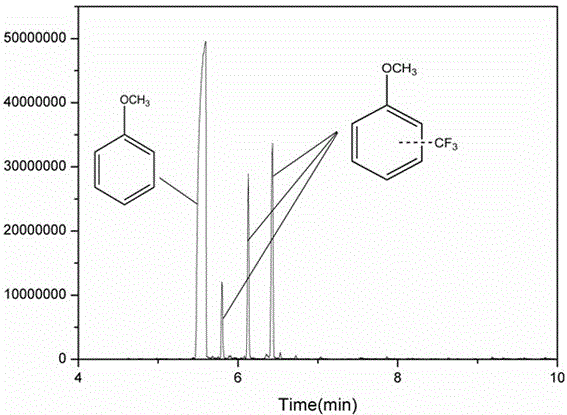
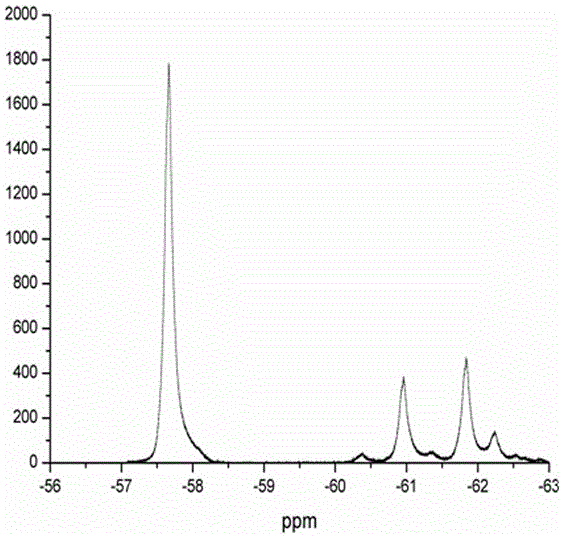
Method for catalyzing arenes or heteroarenes to be subjected to trifluoromethylation by semiconductor photocatalysts
ActiveCN105693480AEliminate complex reaction stepsEasy to operatePhysical/chemical process catalystsOrganic compound preparationTrifluoromethylationCarbon nitride
The invention discloses a method for photo-catalyzing arenes or heteroarenes to be subjected to trifluoromethylation by semiconductor photocatalysts. According to the method, sodium trifluoromethanesulfinate is used as a trifluoromethyl source, acetonitrile is used as a solvent, at the room temperature, visible light is used as driving force, and common semiconductor photocatalysts such as cadmium sulfide, graphite phase carbon nitride and the like are used for catalyzing arenes or heteroarenes to be subjected to trifluoromethylation reactions directly. Raw materials adopted in the method are cheap and easy to obtain, reaction conditions are mild, the method is suitable to be operated in a common atmospheric environment, the methodology for trifluoromethylation of arenes or heteroarenes is enriched, and the method has the potential industrial application value.
Owner:FUZHOU UNIV
Preparation process of trifluoromethyl sulfinyl chloride
The invention provides a preparation process of trifluoromethyl sulfinyl chloride. The preparation process comprises the steps as follows: 1) a synthesis kettle is taken, and thionyl chloride is added; 2) thionyl chloride is heated until reflux and cooled to 0-5 DEG C, and sodium trifluoromethanesulfinate is fed; 3) the temperature is increased to 15-20 DEG C, distillation is performed, the steam is turned off and the temperature is reduced to 10 DEG C or lower when the temperature reaches 90-100 DEG C, and water is added to clean a distillation kettle; 4) the material is distilled into a rectifying still, the rectifying still starts to heat up, front cut fractions are collected after reflux for 3-5 hours, whether the material in the reflux pipe is transparent is observed when the temperature reaches 40-45 DEG C, and a finished product of trifluoromethyl sulfinyl chloride is collected if the material is transparent, and reflux is continued if the material is not transparent; 5) the finished product is stopped from being collected when the temperature reaches 45-50 DEG C, reflux is continued, and the finished product of trifluoromethyl sulfinyl chloride continues to be collected after the reflux temperature is reduced. The process is simple to operate, is green and has no pollutant discharge during preparation, is energy-saving, consumption-reducing and good in economic benefit, an adopted solvent is recyclable, obtained trifluoromethyl sulfinyl chloride has high purity and yield, and the production cost can be effectively reduced.
Owner:JIANGSU TUOQIU AGRI CHEM CO LTD
Trifluoromethanesulfinate purification method
InactiveCN105906536AMeet production requirementsLow costOrganic chemistryPurification methodsSolvent
The invention discloses a trifluoromethanesulfinic acid purification method. The method comprises the following steps: mixing and stirring a sodium trifluoromethanesulfinate industrial product, anhydrous magnesium sulfate and a solvent, carrying out suction filtration while stirring, collecting the filtrate, putting a filter cake in an original stirred reaction kettle, further adding the solvent, carrying out suction filtration while stirring, collecting the filtrate, collecting all the filtrates and filtrate solutions after leaching and putting all the filtrates and filtrate solutions after leaching in the kettle, carrying out reduced-pressure drying, recovering the solvent, adding deionized water to obtain a sodium trifluoromethanesulfinate water solution; then sending the sodium trifluoromethanesulfinate water solution to a dryer for spray drying, thus obtaining the sodium trifluoromethanesulfinate powder product with content of 95% or above. The method has the beneficial effects that high content sodium trifluoromethanesulfinate is extracted from the sodium trifluoromethanesulfinate industrial product by using the solvent and high content sodium trifluoromethanesulfinate without inorganic salts, such as bromide ions, is obtained after the solvent is removed, thus meeting the production requirements of lithium ion battery electrolyte bis(trifluoromethane)sulfonimide lithium salt.
Owner:YANCHENG INST OF TECH
Preparation method of trifluoromethylated 2,4-quinolinedione compound
ActiveCN114014805AMild reaction conditionsEasy to solveOrganic chemistryChemical recyclingTrifluoromethylationMeth-
The invention discloses a preparation method of a trifluoromethylated 2,4-quinolinedione compound and a preparation method of the trifluoromethylated 2,4-quinolinedione compound. The preparation method comprises the following steps: adding an N-o-cyano aryl acrylamide compound, sodium trifluoromethanesulfinate, a photocatalyst and a solvent into a reactor; stirring and reacting the mixture under the conditions of air atmosphere and visible light irradiation; after the reaction is completed, concentrating the product, and separating by silica gel column chromatography to obtain the trifluoromethylated 2,4-quinolinedione compound after the reaction is completed. According to the method, transition metal catalysis is not needed, the reaction can be carried out at room temperature, oxygen in air is used as an oxidizing agent, and other oxidizing agents with strong oxidizing property can be prevented from being additionally added.
Owner:GUANGXI TEACHERS EDUCATION UNIV
Method for converting benzene into benzotrifluoride through heterogeneous catalysis
ActiveCN105585418ALow costMild reaction conditionsHalogenated hydrocarbon preparationHydration reactionTrifluoromethylation
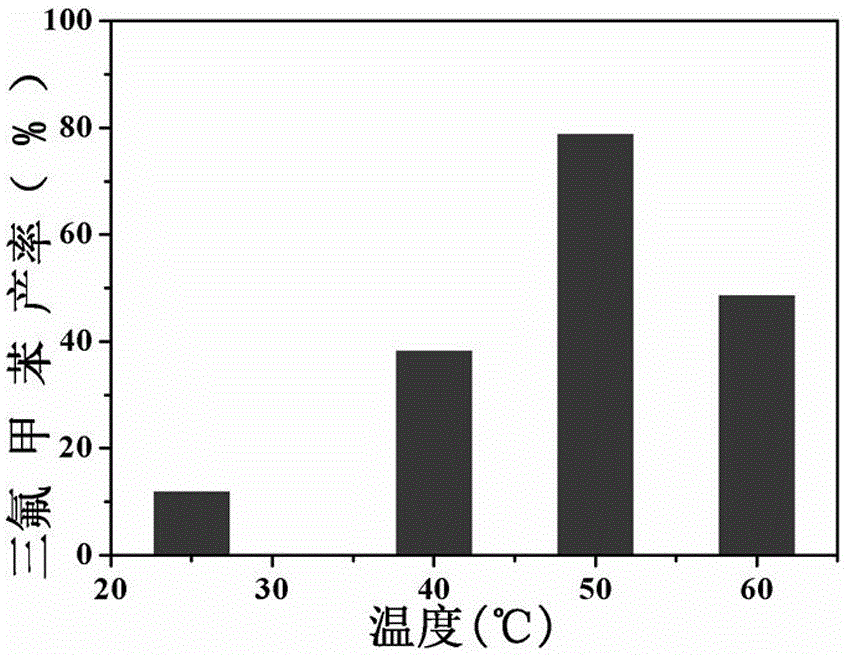
The invention discloses a method for converting benzene into benzotrifluoride through heterogeneous catalysis. Benzene is catalyzed to generate benzotrifluoride at lower temperature and normal pressure with manganese sulfate monohydrate as a catalyst, sodium trifluoromethanesulfinate as a trifluoromethyl source and acetonitrile as solvent. The raw materials are low in price and easy to obtain, conditions are mild, environment pollution is low, products are easy to separate, hydrogen fluoride, sulfur tetrafluoride and other highly-corrosive and highly-toxic fluorinated reagents used in current industrial trifluoromethylation reactions are avoided, large-scale industrial production is facilitated, and the method can be further used for trifluoromethyl modification of organic compounds with biological activity and medicine activity and has good application prospects and economic benefits.
Owner:FUZHOU UNIV
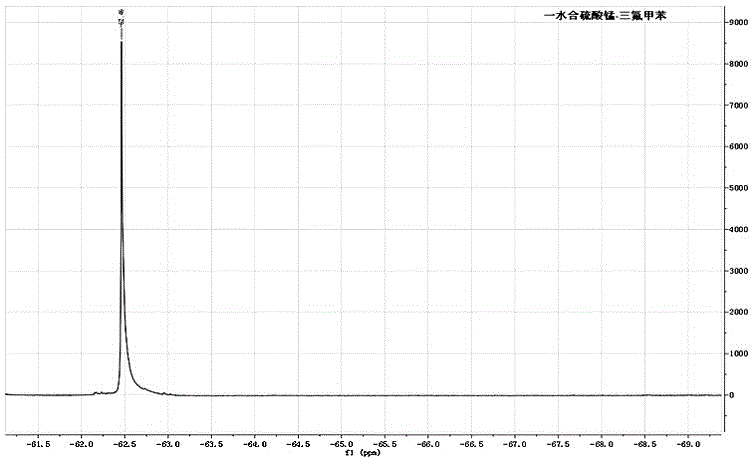
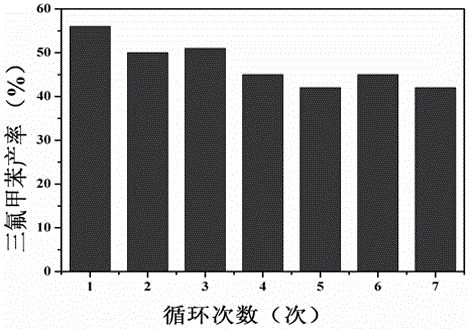
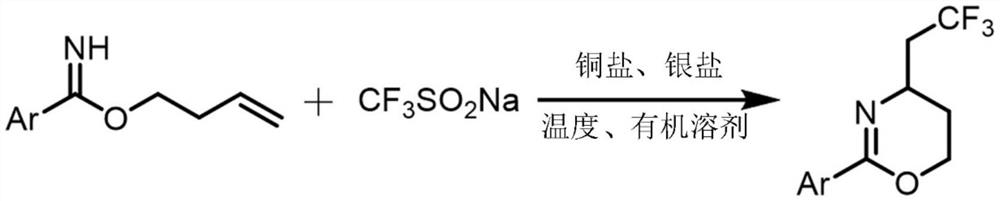
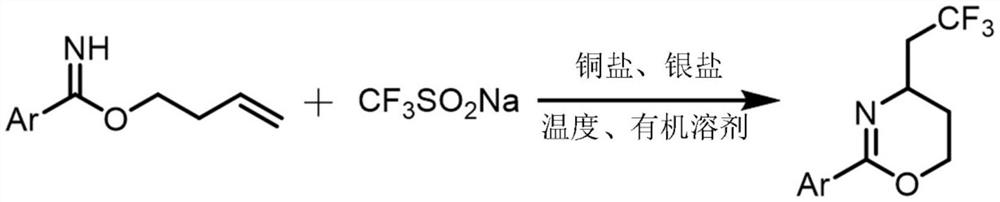
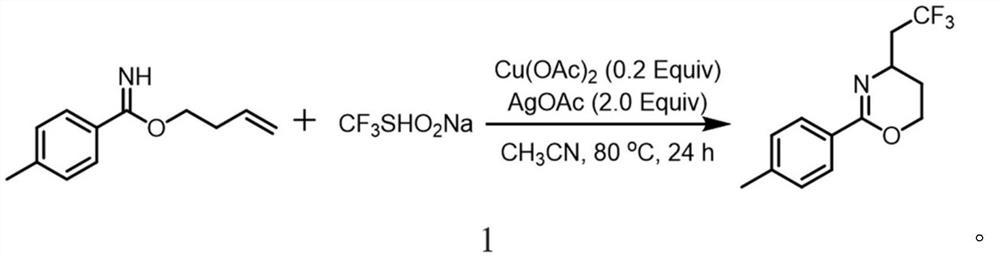
Synthesis method of trifluoromethylated 1, 3-oxazine compound
The invention relates to a synthetic method of a trifluoromethylated 1, 3-oxazine compound, which comprises the following steps: 1) selecting aryl formamide homoallyl ester, sodium trifluoromethanesulfinate, silver salt and copper salt, and sequentially adding into an organic solvent; and 2) heating to a proper temperature to prepare the trifluoromethyl substituted 1, 3-oxazine compound. Accordingto the method, aryl formamide homoallyl ester is used as a substrate, cheap sodium trifluoromethanesulfinate is used as a trifluoromethyl reagent, the trifluoromethyl substituted 1, 3-oxazine compound is synthesized under the action of copper salt and silver salt, and an economic synthesis route is provided for the compound.
Owner:ZUNYI MEDICAL UNIVERSITY
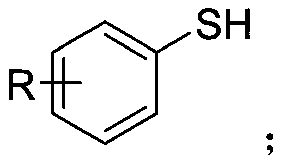
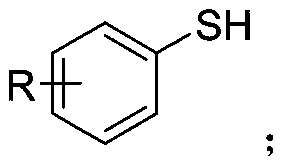
Synthetic method of aryl trifluoromethylthio compound
ActiveCN111235594AMild conditionsSimple and safe operationElectrolysis componentsElectrolytic organic productionSupporting electrolyteAryl
The invention discloses a synthesis method of an aryl trifluoromethylthio compound. Aryl thiophenol and sodium trifluoromethanesulfinate are adopted as raw materials, constant-current electrolysis isperformed in the presence of a supporting electrolyte to implement coupling of carbon-sulfur bonds, and separation and purification are performed to obtain the aryl trifluoromethylthio compound. According to the method, an electrochemical synthesis method is applied, the aryl trifluoromethylthio compound with high additional value is synthesized from cheap and easily available raw materials, the reaction conditions are mild, green and efficient, and a metal catalyst and an oxidizing agent are not required to be used.
Owner:HEFEI UNIV OF TECH
2-trifluoroalkyl-1, 4-naphthoquinone compound and synthesis method thereof
PendingCN114702399ALow priceEasy to storeOrganic chemistryOrganic compound preparationPotassium persulfatePtru catalyst
The invention relates to a 2-trifluoroalkyl-1, 4-naphthoquinone compound and a synthesis method thereof, and the synthesis method specifically comprises the following steps: dispersing olefin with a structure (I), 1, 4-naphthoquinone with a structure (II), sodium trifluoromethanesulfinate with a structure (III), potassium persulfate and a catalyst in a solvent, and stirring and heating to construct a 2-trifluoroalkyl-1, 4-naphthoquinone target compound with a structure (IV). The invention provides a novel method for synthesizing a 2-trifluoroalkyl-1, 4-naphthoquinone compound by taking olefin, 1, 4-naphthoquinone and sodium trifluoromethanesulfinate as initial materials for reaction, iron salt as a catalyst and potassium persulfate as an oxidant for the first time. The substrate, the ferric salt catalyst and the potassium peroxodisulfate oxidant used in the method are low in price and easy to store, the method has the advantages of low production cost, simple operation, wide substrate range, product diversity and the like, and gram-scale preparation can be realized.
Owner:XINYANG NORMAL UNIVERSITY
Preparation method of N-(2-methylthiophenyl)isoindole-1,3-diketone compound
ActiveCN108191737ASimple processSimple and fast operationOrganic chemistry methodsDiketoneRotary evaporator
The invention discloses a preparation method of an N-(2-methylthiophenyl)isoindole-1,3-diketone compound. The preparation method comprises the following steps: by taking N-(2-methylthiophenyl)benzamide as a substrate; adding sodium trifluoromethanesulfinate into the substrate as a carbonyl source; adding a catalyst and carrying out stirred reaction in a reaction solvent for 24h under normal pressure and at 120 DEG C; after reaction is finished, filtering a reaction solution to obtain filtrate; concentrating the filtrate and removing the solvent by utilizing the rotary evaporator, so as to obtain residues; carrying out chromatography on the residues through a silica gel column; eluting through an eluting solution and collecting effluent liquid according to an actual gradient; combining theeffluent liquid containing a product, concentrating the combined effluent liquid and removing the solvent; finally, drying in vacuum to obtain a target product. The preparation method disclosed by theinvention has the advantages of simple preparation technology, less pollution, low energy consumption and high yield.
Owner:WENZHOU UNIVERSITY
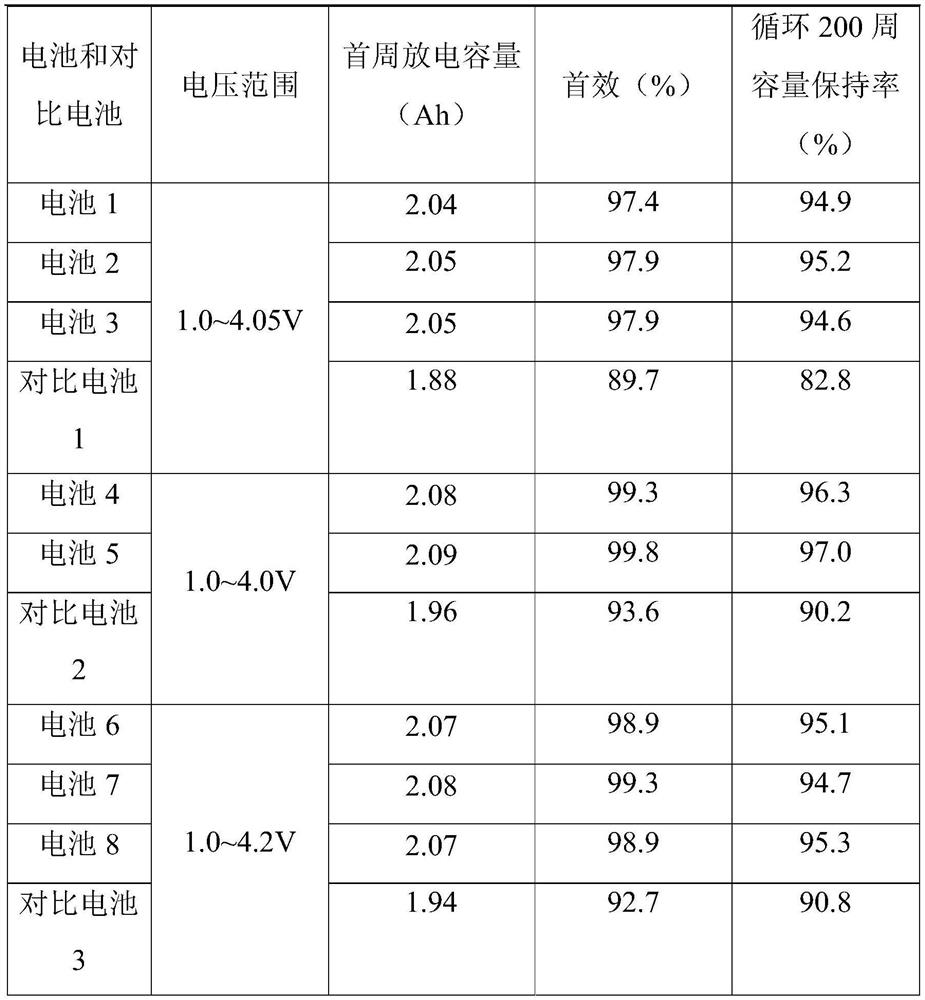
Sodium ion battery
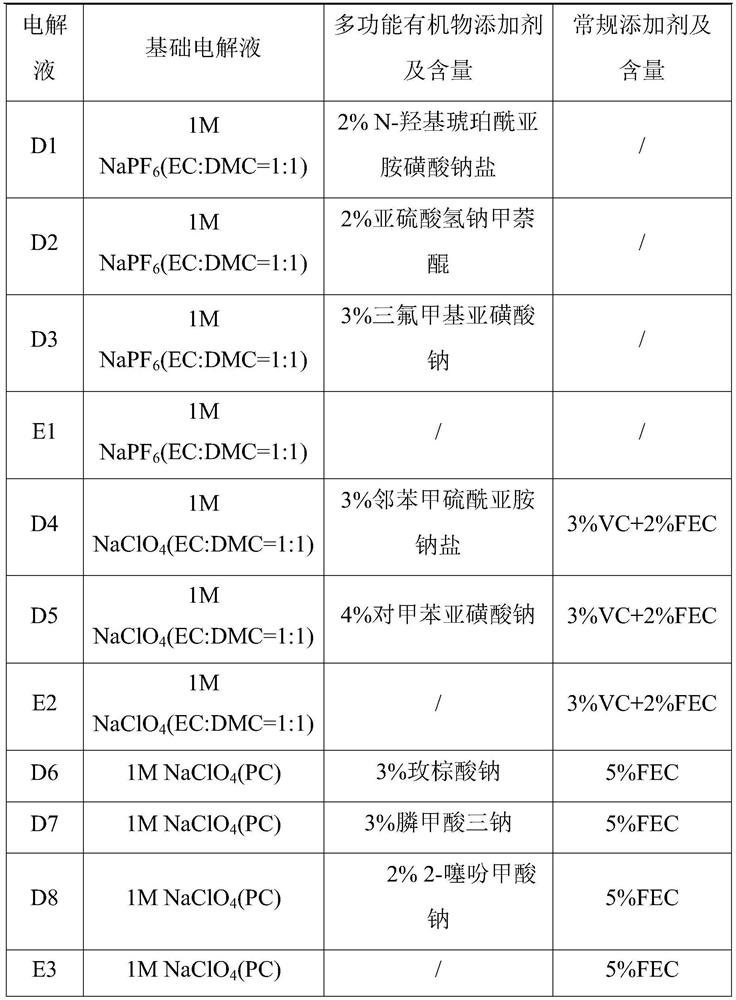
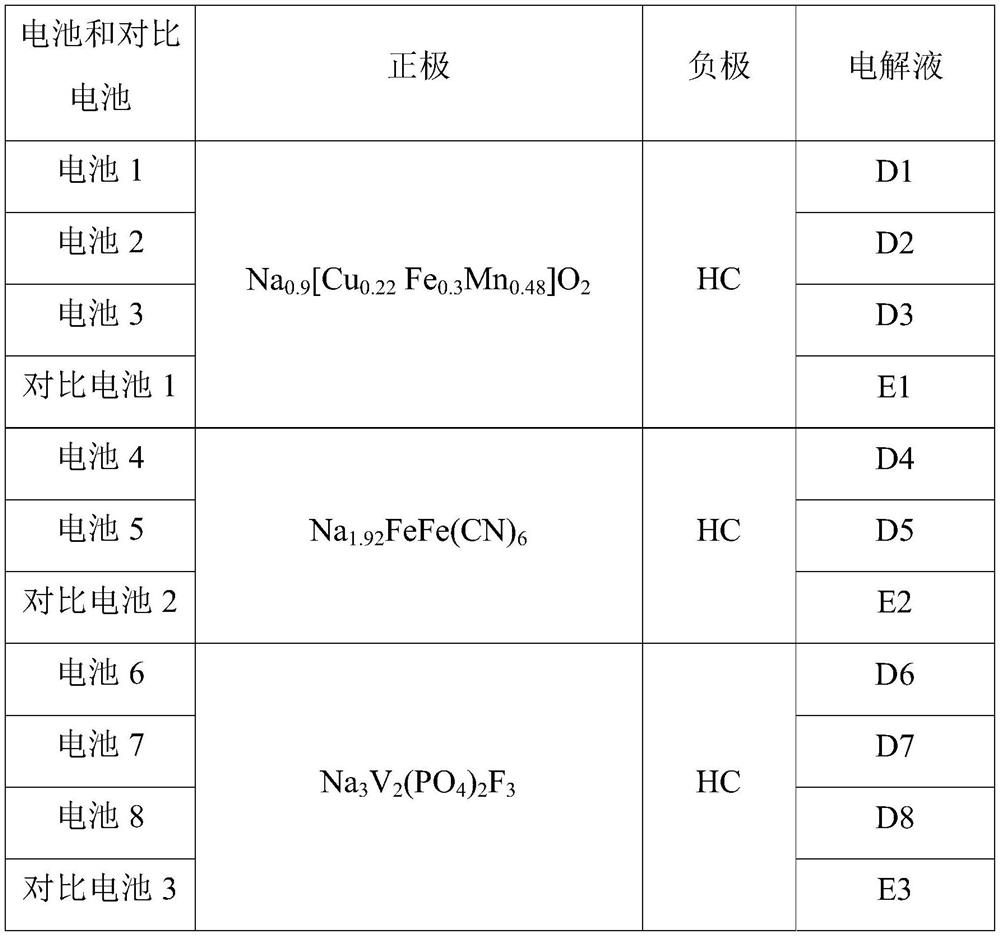
PendingCN114447333AImprove utilization efficiencyImprove the first effectSecondary cellsNon-aqueous electrolyte accumulator electrodesElectrolytic agentSodium-ion battery
The invention provides a sodium-ion battery, which comprises a sodium-ion battery electrolyte and a sodium-ion battery positive electrode, and the sodium-ion battery electrolyte and / or the sodium-ion battery positive electrode contain / contains a multifunctional organic matter additive, the multifunctional organic matter additive is one or more of N-hydroxysuccinimide sodium sulfonate, menadione sodium bisulfite, sodium trifluoromethanesulfinate, o-phenylmethimide sodium salt, sodium p-toluenesulfinate, sodium rose palmate, trisodium phosphinate and 2-thiophenic acid sodium salt. The multifunctional organic matter additive has the beneficial effects that the multifunctional organic matter additive has sodium supplementing and film forming effects at the same time, and the remaining multifunctional organic matter additive can also be used as electrolyte sodium salt. The multifunctional organic matter additive can improve the cycle performance of the battery at room temperature and high temperature while improving the first efficiency, the discharge capacity and the energy density of the battery, and the high temperature resistance of the sodium ion battery is improved.
Owner:天津中电新能源研究院有限公司
A kind of chroman-4-one compound containing trifluoromethyl group and preparation method thereof
The invention provides a kind of allyl salicylaldehyde (I) compound as the starting material for the reaction, sodium trifluoromethyl sulfinate (II) as the trifluoromethyl source and persulfate as the oxidant, The trifluoromethyl-containing chroman-4-one target compound (III) and its preparation method. The method involves mild reaction conditions, simple operation, product diversity, and can realize gram-scale production.
Owner:XINYANG NORMAL UNIVERSITY
Synthetic method for electrochemical oxidation of 2-trifluoromethyl-alpha-carbonyl ketene dithioacetal compound
PendingCN114214646AMild reaction conditionsEasy to operateElectrolysis componentsElectrolytic organic productionMeth-Organic synthesis
The invention relates to the technical field of organic synthesis, in particular to a synthetic method for electrochemical oxidation of a 2-trifluoromethyl-alpha-carbonyl ketene dithioacetal compound, which comprises the following steps: A, sequentially adding a compound 1, a compound 2, an electrolyte and a solvent into a reactor equipped with an electrode plate; b, carrying out stirring reaction on the compound 1 and the compound 2 under the conditions of certain temperature and constant current under an open condition; c, after the reaction is finished, decompressing and evaporating to remove the solvent to obtain a crude product; and D, carrying out column chromatography purification to obtain a 2-trifluoromethyl-alpha-carbonyl ketene dithioacetal compound 3. According to the method, alpha-carbonyl ketene dithioacetal and sodium trifluoromethanesulfinate are used as raw materials, an anode electrode slice is a carbon felt electrode, a cathode electrode slice is an iron sheet electrode, the energizing current is 10 milliamperes, an electrolyte is a solvent formed by mixing tetra-n-butylammonium perchlorate and acetonitrile / water, and the reaction temperature is room temperature; the 2-trifluoromethyl-alpha-carbonyl ketene dithioacetal compound can be efficiently synthesized by the method; compared with a traditional synthesis method, the method has the advantages that the reaction condition is mild, and the method can be smoothly carried out at room temperature; the operation is simple, and all operations can be carried out in an open system; current is used as an oxidation method in the reaction, so that pollution of transition metal and a chemical oxidant is avoided; the raw materials are easy to obtain, the functional group compatibility is good, and the substrate application range is wide.
Owner:NANTONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
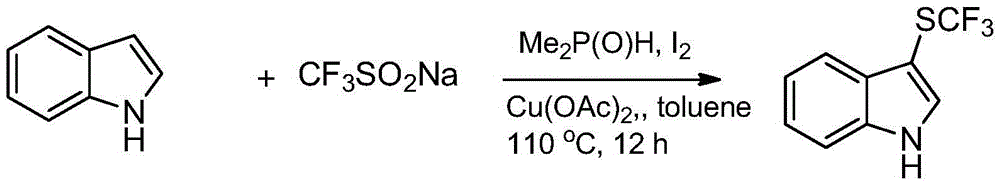
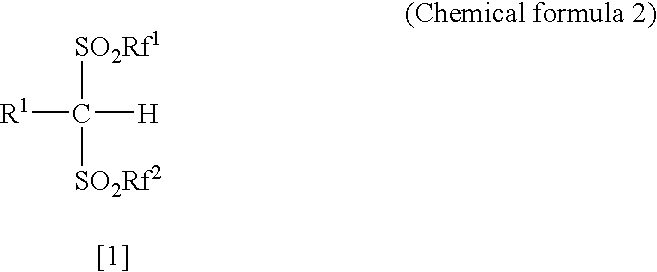

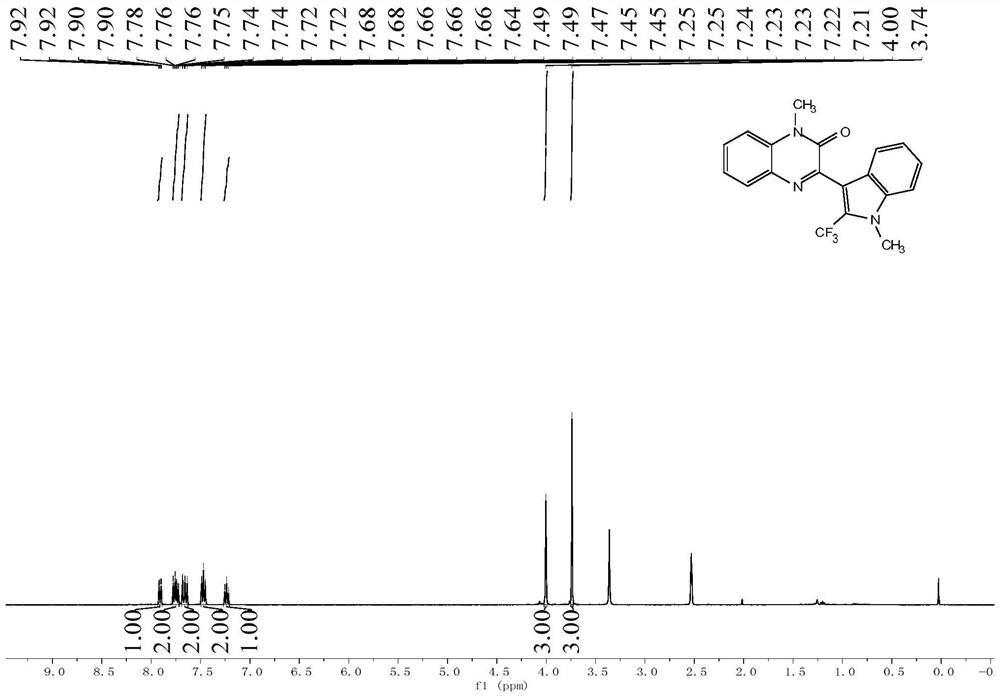
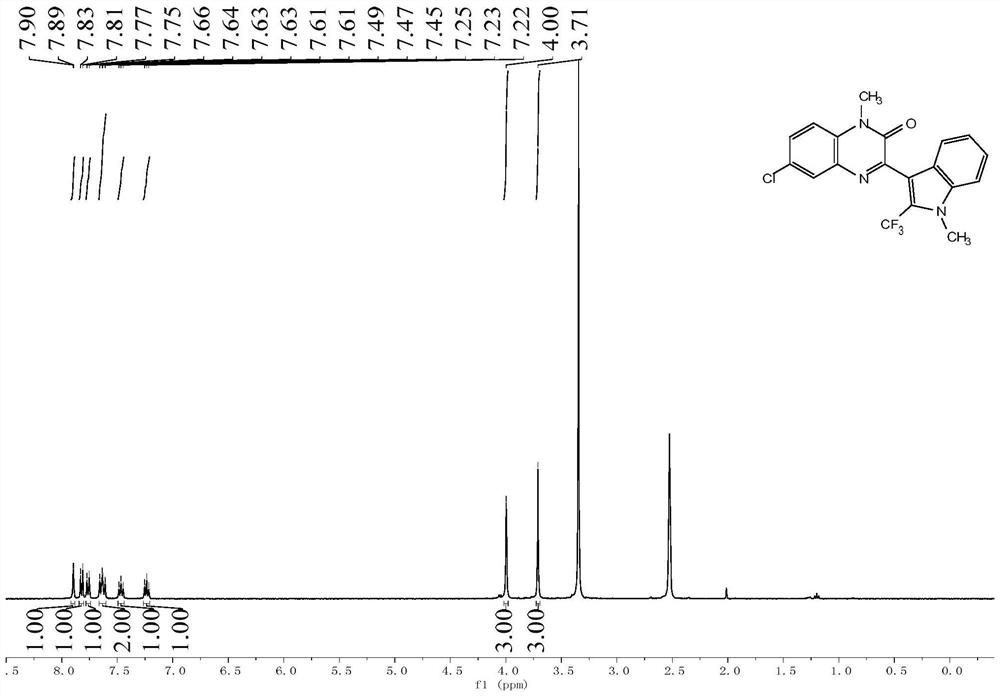
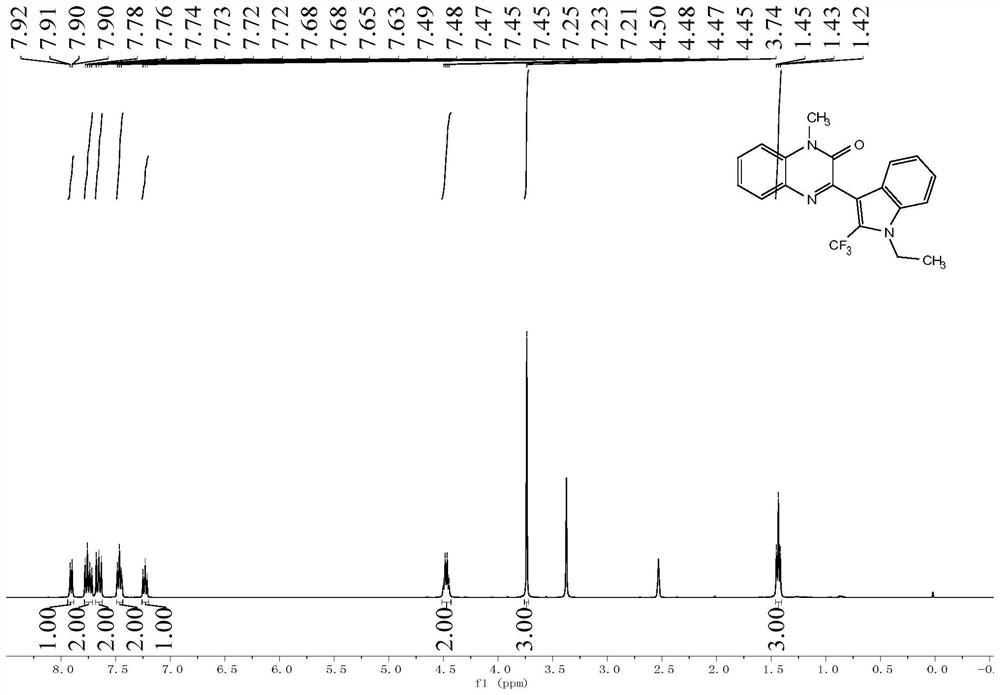
![Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol](https://images-eureka.patsnap.com/patent_img/98149c03-0d2f-4141-b10d-b0305e56df9d/BDA0002401526650000041.png)
![Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol](https://images-eureka.patsnap.com/patent_img/98149c03-0d2f-4141-b10d-b0305e56df9d/BDA0002401526650000042.png)
![Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol Preparation method of visible-light-catalyzed [beta]-trifluoromethyl alcohol](https://images-eureka.patsnap.com/patent_img/98149c03-0d2f-4141-b10d-b0305e56df9d/BDA0002401526650000051.png)
![Synthesis method of bis [(trifluoromethyl) sulfonyl] methane Synthesis method of bis [(trifluoromethyl) sulfonyl] methane](https://images-eureka.patsnap.com/patent_img/a0450023-7b62-4ddb-a657-26c30c005f97/HDA0001916500730000011.png)
![Synthesis method of bis [(trifluoromethyl) sulfonyl] methane Synthesis method of bis [(trifluoromethyl) sulfonyl] methane](https://images-eureka.patsnap.com/patent_img/a0450023-7b62-4ddb-a657-26c30c005f97/BDA0001916500720000071.png)
![Synthesis method of bis [(trifluoromethyl) sulfonyl] methane Synthesis method of bis [(trifluoromethyl) sulfonyl] methane](https://images-eureka.patsnap.com/patent_img/a0450023-7b62-4ddb-a657-26c30c005f97/BDA0001916500720000072.png)