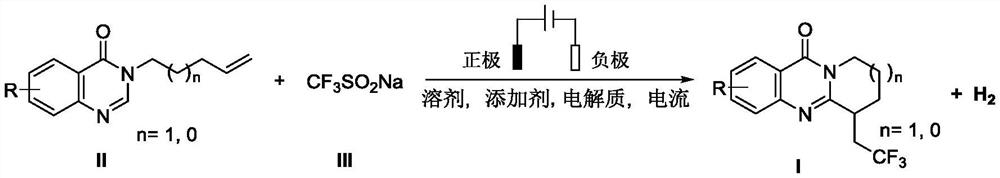
Electrochemical synthesis method of trifluoromethyl-substituted polycyclic quinazolinone derivative
A polycyclic quinazolinone and trifluoromethyl technology, which is applied in the field of electrochemical synthesis of polycyclic quinazolinone derivatives, can solve the problems of large amount of three wastes, harsh reaction conditions, and less synthesis, and achieve simple operation , Raw materials are easily available, and the effect of environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
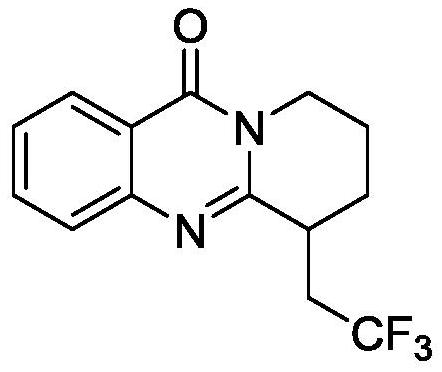
[0020] Example 1 6-(2,2,2-trifluoroethyl)-6,7,8,9-tetrahydro-11H-pyridin[2,1-b]quinazolin-11-one (I-a) preparation
[0021] Put 3-(pent-4-en-1-yl)quinazolin-4(3H)-one (II-a) (43mg, 0.2mmol) into the Shrek tube, sodium trifluoromethylsulfinate ( 94mg, 0.6mmol), p-toluenesulfonic acid (38mg, 0.2mmol), potassium hexafluorophosphate (35mg, 0.2mmol). A graphite felt anode (1.5×1×0.5 cm) and a nickel foam cathode (1.5×1 cm) were inserted in the reactor. Add dimethyl sulfoxide (4.5mL) and water (1.5mL) to form a mixed solvent, adjust the current to a constant current of 6mA, react at 60°C for 12 hours, and separate by silica gel column chromatography to obtain a white solid I-a, 42mg, m.p.143.2- 144.6°C, yield: 74%. The structural formula of I-a is:
[0022]
[0023] 1 H NMR (600MHz, CDCl 3 )δ8.22(dd, J=7.8,1.8Hz,1H),7.69(m,1H),7.59(dd,J=7.8,1.2Hz,1H),7.45–7.36(m,1H),7.31–4.27 (m,1H),3.92(dt,J=13.2,6.6Hz,1H),3.56–3.47(m,1H),3.18–3.43(m,1H),2.45–2.29(m,2H),2.07–1.94 (m,2H),...
Embodiment 2
[0024] Example 2 6-(2,2,2-trifluoroethyl)-6,7,8,9-tetrahydro-11H-pyridin[2,1-b]quinazolin-11-one (I-a) Preparation Put 3-(pent-4-en-1-yl)quinazolin-4(3H)-one (II-a) (43mg, 0.2mmol) and sodium trifluoromethylsulfinate into Shrek tube (94mg, 0.6mmol), benzoic acid (24mg, 0.2mmol), potassium hexafluorophosphate (35mg, 0.2mmol).
[0025] A graphite felt anode and a nickel foam cathode were inserted into the reactor. Dimethyl sulfoxide (4.5 mL) and water (1.5 mL) were added to form a mixed solvent. The current was adjusted to a constant current of 6mA, reacted at 60°C for 12 hours, and separated by silica gel column chromatography to obtain a white solid I-a, 32 mg, yield: 55%.
Embodiment 3
[0026] Example 3 6-(2,2,2-trifluoroethyl)-6,7,8,9-tetrahydro-11H-pyridin[2,1-b]quinazolin-11-one (I-a) preparation
[0027] Put 3-(pent-4-en-1-yl)quinazolin-4(3H)-one (II-a) (43mg, 0.2mmol) into the Shrek tube, sodium trifluoromethylsulfinate ( 94mg, 0.6mmol), acetic acid (12mg, 0.2mmol,), potassium hexafluorophosphate (35mg, 0.2mmol).
[0028] A graphite felt anode and a nickel foam cathode were inserted into the reactor. Dimethyl sulfoxide (4.5 mL) and water (1.5 mL) were added to form a mixed solvent. The current was adjusted to a constant current of 6mA, reacted at 60°C for 12 hours, and separated by silica gel column chromatography to obtain a white solid I-a, 38 mg, yield: 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com