Synthetic method for electrochemical oxidation of 2-trifluoromethyl-alpha-carbonyl ketene dithioacetal compound
A carbonyl dithioketal and trifluoromethyl technology are applied in the synthesis field of electrochemical oxidation of 2-trifluoromethyl-α-carbonyl dithioketal compounds, and achieve the advantages of avoiding pollution, low cost and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] refer to Figure 1-Figure 16 , a kind of synthetic method of electrochemical oxidation 2-trifluoromethyl-alpha-carbonyl dithioketene compound, comprises the following steps:
[0043] A, sequentially add compound 1, compound 2, electrolyte and solvent in the reactor equipped with electrode sheet;
[0044] B, under open conditions, above-mentioned compound 1 and compound 2 are carried out stirring reaction under the condition of certain temperature and constant electric current;
[0045] After C, reaction finishes, decompression evaporates solvent and obtains crude product;
[0046] D. Purify by column chromatography to obtain 2-trifluoromethyl-α-carbonyl dithioketene compound 3.
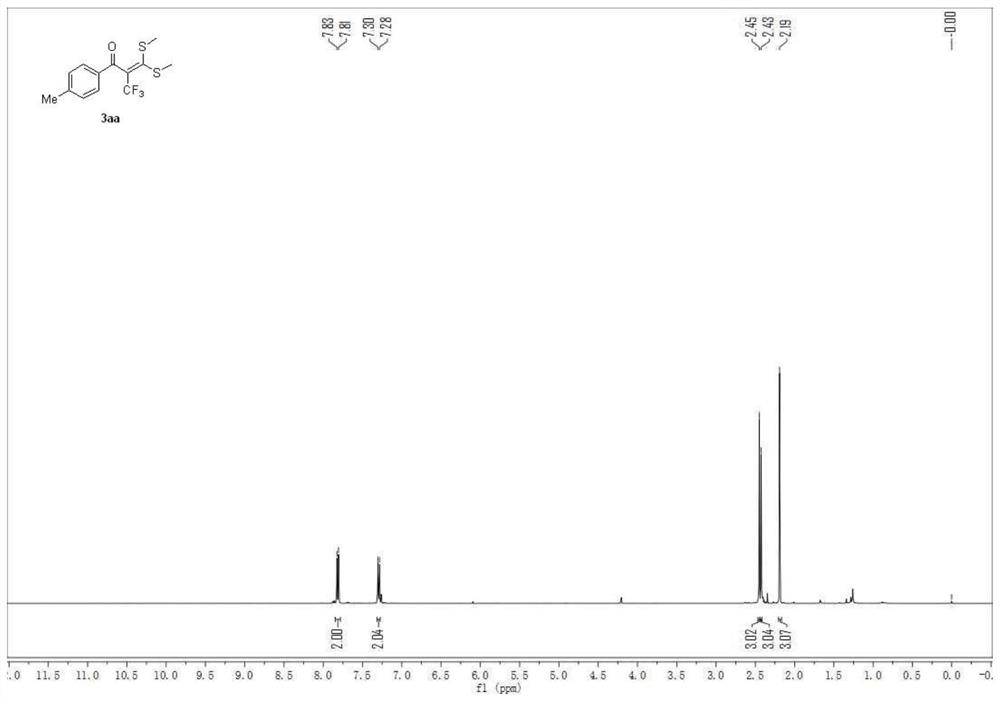
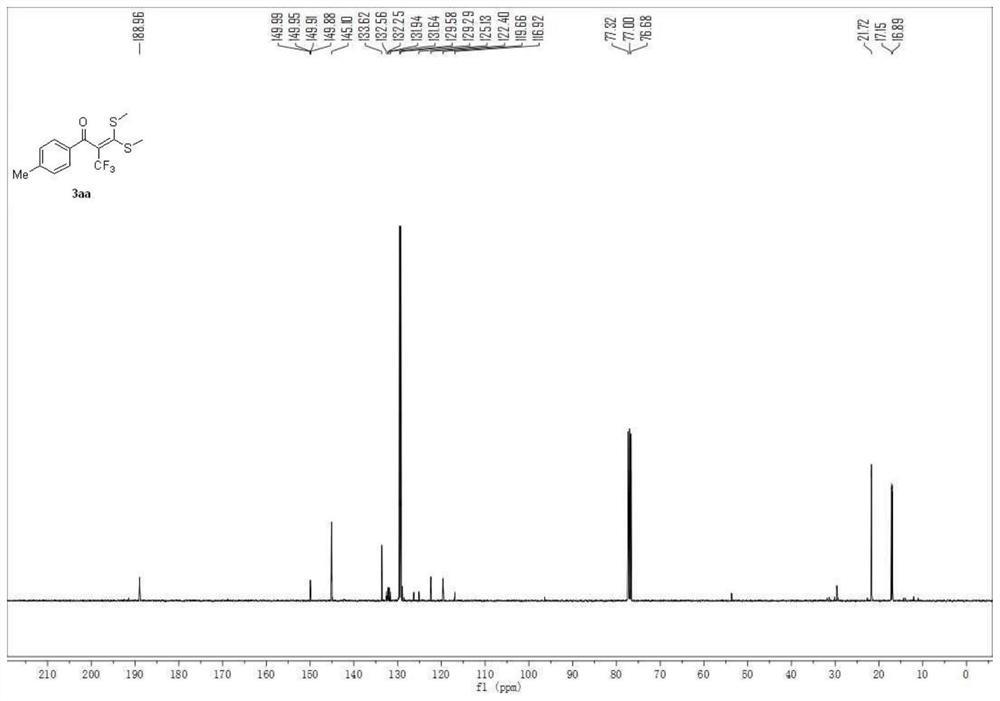
Embodiment 1
[0048] 3,3-bis(methylthio)-1-(p-tolyl)-2-(trifluoromethyl)prop-2-en-1-one
[0049]
[0050] A carbon felt sheet (size: 10mm×10mm×0.3mm) was assembled as an anode and an iron sheet (size: 10mm×10mm×0.3mm) was used as a cathode in a 10mL electrolytic cell without a diaphragm, and compound 1 (α-carbonyl disulfide Ketal, 0.5mmol), compound 2 (sodium trifluoromethylsulfinate, 1.5mmol, 234mg), electrolyte tetra-n-butylammonium perchlorate (1.5mmol, 513mg), water 1mL, acetonitrile 6mL at constant current The reaction was stirred at room temperature under 10 mA for 4 hours. After the reaction, the resulting mixture was poured into 15 mL of water and extracted with dichloromethane (3×15 mL). The combined organic layers were washed with 15 mL of brine and dried over anhydrous sodium sulfate. The solvent was removed on a rotary evaporator, and the residue was purified by flash chromatography [silica gel, petroleum ether: ethyl acetate volume ratio (50-100): 1] to obtain 114.3 mg of the...
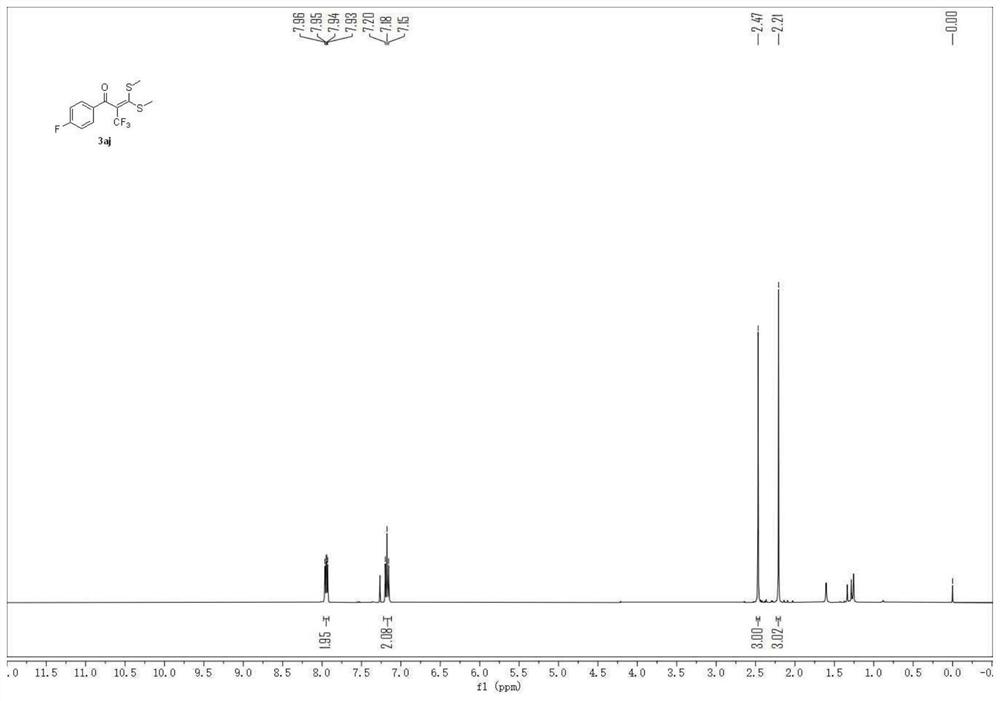
Embodiment 2
[0052] 1-(4-fluorophenyl)-3,3-bis(methylthio)-2-(trifluoromethyl)prop-2-en-1-one
[0053]
[0054] A carbon felt sheet (size: 10mm×10mm×0.3mm) was assembled as an anode and an iron sheet (size: 10mm×10mm×0.3mm) was used as a cathode in a 10mL electrolytic cell without a diaphragm, and compound 1 (α-carbonyl disulfide Ketal, 0.5mmol), compound 2 (sodium trifluoromethylsulfinate, 1.5mmol, 234mg), electrolyte tetra-n-butylammonium perchlorate (1.5mmol, 513mg), water 1mL, acetonitrile 6mL at constant current The reaction was stirred at room temperature under 10 mA for 4 hours. After the reaction, the resulting mixture was poured into 15 mL of water and extracted with dichloromethane (3×15 mL). The combined organic layers were washed with 15 mL of brine and dried over anhydrous sodium sulfate. The solvent was removed on a rotary evaporator, and the residue was purified by flash chromatography [silica gel, petroleum ether: ethyl acetate volume ratio (50-100): 1] to obtain 81.3 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com