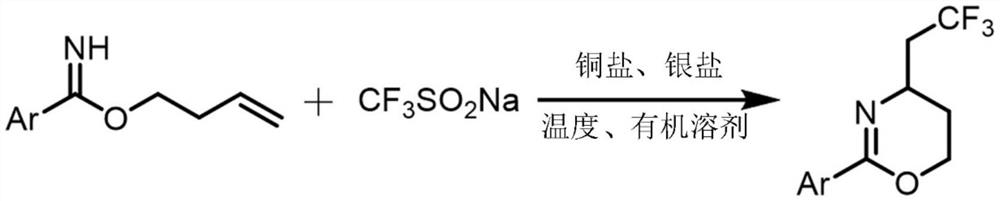
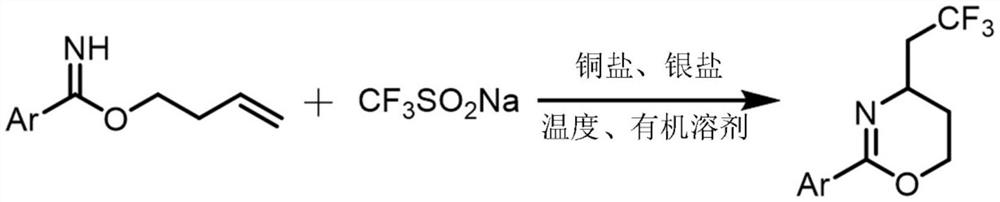
Synthesis method of trifluoromethylated 1, 3-oxazine compound
A technology of trifluoromethylation and synthesis method, which is applied in the field of synthesis of trifluoromethylated 1,3-oxazine compounds, and can solve the problems of poor reaction atom economy and limited industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
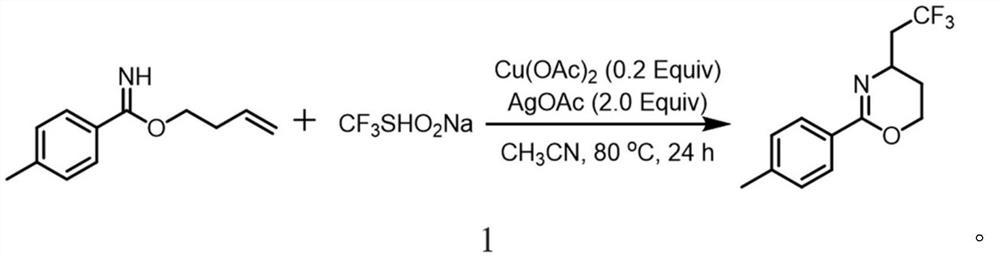
[0023] A method for synthesizing trifluoromethylated 1,3-oxazine compounds, comprising the following steps: 1) Using p-toluimide homoallyl ester as a substrate, sodium trifluoromethanesulfinate Is a trifluoromethyl reagent, copper acetate is a copper salt, silver acetate is a silver salt, and acetonitrile is a reaction solvent;
[0024] 2) Combine p-methylbenzimide homoallyl ester (37.8mg, 0.2mmol), sodium trifluoromethanesulfinate (62.4mg, 0.4mmol), silver acetate (66.8mg, 0.4mmol), acetic acid Copper (7.2mg, 0.04mmol) was sequentially added to stirring acetonitrile (2mL), and reacted at 80°C for 24 hours. After extraction, drying, concentration and silica gel column chromatography, a colorless liquid (18.2mg, 71%) was obtained. , The chemical reaction formula 1 is:
[0025]
[0026] The product testing data is as follows:
[0027] 1 H NMR(400MHz, CDCl 3 )δ7.78(d,J=8.3Hz,2H), 7.16(d,J=8.0Hz,2H), 4.49–4.36(m,1H), 4.34–4.21(m,1H), 3.92–3.81(m ,1H), 2.76–2.65(m,1H), 2.38(s,3H), 2.25...
Embodiment 2
[0029] A method for synthesizing trifluoromethylated 1,3-oxazine compounds, comprising the following steps: 1) Using p-toluimide homoallyl ester as the substrate, silver benzoate as the carboxyl reagent, and acetic acid Copper is a copper salt, and acetonitrile is a reaction solvent;
[0030] 2) Combine p-methylbenzimide homoallyl ester (44.5mg, 0.2mmol), sodium trifluoromethanesulfinate (62.4mg, 0.4mmol), silver acetate (66.8mg, 0.4mmol), acetic acid Copper (7.2mg, 0.04mmol) was sequentially added to stirring acetonitrile (2mL), reacted at 80°C for 24 hours, and then extracted, dried, concentrated and silica gel column chromatography was used to obtain a colorless liquid (37.5mg, 64%) , The chemical reaction formula 2 is:
[0031]
[0032] The product testing data is as follows:
[0033] 1 H NMR(400MHz, CDCl 3 )δ8.40(s,1H), 8.04(d,J=8.6Hz,1H),7.93-7.89(m,1H),7.85(t,J=7.1Hz,2H),7.57-7.44(m,2H ), 4.56–4.43(m,1H), 4.41–4.34(m,1H), 4.03–3.92(m,1H), 2.89–2.66(m,1H), 2.37–2.24(m,2H), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com