Method for converting benzene into benzotrifluoride through heterogeneous catalysis
A technology of trifluorotoluene and catalytic benzene, which is applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation, etc., can solve problems such as high cost and high pollution, achieve cost reduction, simple reaction process, and is conducive to large-scale the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Weigh 0.2 mmol of manganese sulfate monohydrate as catalyst, 0.5 mmol of sodium trifluoromethyl sulfinate as trifluoromethyl source, mix in the reactor, add 1 mL of acetonitrile as solvent and 0.5 mL of reactant benzene;
[0025] (2) Put the reactor into an ultrasonic cleaning machine for 50KHz ultrasonic treatment for 30s, connect the reactor to an air balloon, and place it in a heat-collecting constant temperature magnetic stirrer at 50°C for 24 hours;
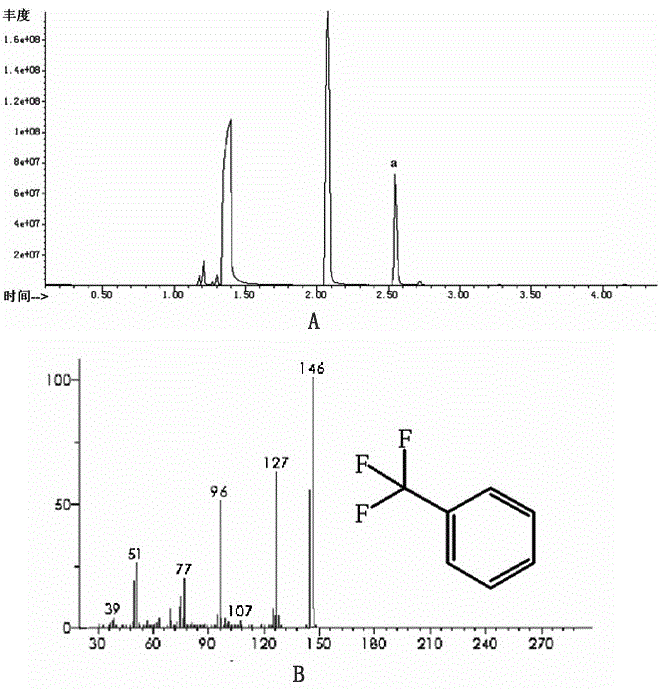
[0026] (3) Centrifuge after the reaction is complete, and detect the supernatant with a GC-MS5977A mass spectrometer. The chromatographic column used is HP-5MS (5% polymethylsilane fixed solution, the specification is 30m×0.32mm×0.25μm), and the quantitative analysis is performed by the FID detector of GCAgilent7890B chromatograph. The GC-MS spectrogram of the supernatant obtained by the reaction (see figure 1 -A), after matching ( figure 1 -B) After that, the peak with a retention time of 2.5min (a) is triflu...
Embodiment 2
[0028] (1) Weigh 0.2 mmol of manganese sulfate monohydrate as catalyst, 0.5 mmol of sodium trifluoromethyl sulfinate as trifluoromethyl source, mix in the reactor, add 1 mL of acetonitrile as solvent and 0.5 mL of reactant benzene;
[0029] (2) Put the reactor into an ultrasonic cleaning machine for 70KHz ultrasonic treatment for 30s, connect the reactor to an air balloon, and place it in a heat-collecting constant temperature magnetic stirrer at 50°C for 24 hours;
[0030] (3) Centrifuge after the reaction is complete, and detect the supernatant with a GC-MS5977A mass spectrometer;
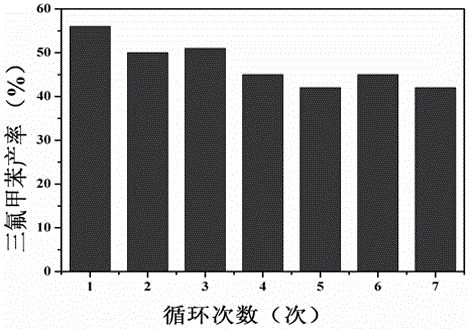
[0031] (4) Take the precipitate after centrifugation in step (3), wash it with acetonitrile for 3 times, put it in a vacuum oven and dry it at 60°C, and then conduct a recycling experiment on the dried manganese sulfate monohydrate, that is, repeat the steps 7 times (1 )-(4) operation.
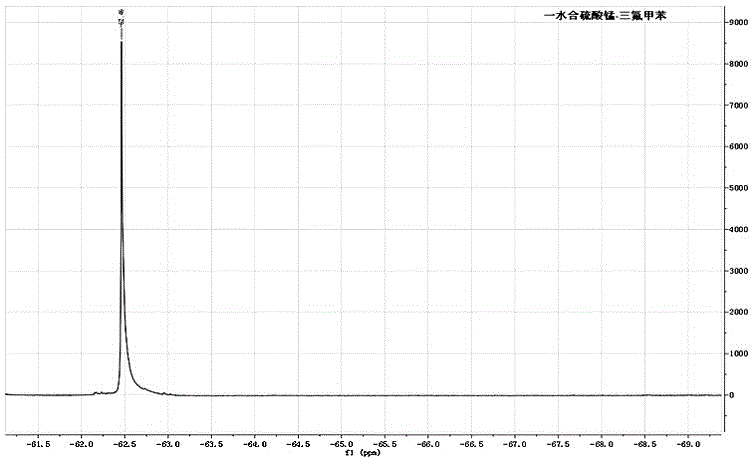
[0032] The results show that the mass spectrum and nuclear magnetic resonance spectra of the supernatant obta...
Embodiment 3
[0034] (1) Weigh 0.2 mmol of manganese sulfate monohydrate as catalyst, 0.5 mmol of sodium trifluoromethyl sulfinate as trifluoromethyl source, mix in the reactor, add 1 mL of acetonitrile as solvent and 0.5 mL of reactant benzene;
[0035] (2) Put the reactor into an ultrasonic cleaning machine for 60KHz ultrasonic treatment for 30s, connect the reactor to an air balloon, and place it in a heat-collecting constant temperature magnetic stirrer at 50°C for 6, 12, 18, and 24 hours respectively.
[0036] By the change relationship figure of different reaction times and trifluorotoluene productive rate (see Figure 4 ) It can be seen that with the prolongation of the reaction time, the yield of benzotrifluoride increases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com