Preparation method of alpha-trifluoromethylthio substituted acetophenone compound
A technology of trifluoromethylthio and acetophenone, which is applied in the preparation of thioether, the formation/introduction of thiol/thioether, organic chemistry, etc. , the use of expensive and other problems, to achieve the effect of simple and convenient preparation method, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention is a kind of preparation method of α-trifluoromethylthio substituted acetophenone compound, and the steps comprise:
[0061] Aryl methyl ketone as substrate, sodium trifluoromethanesulfinate as trifluoromethylthio reagent, triphosgene as reaction additive, pyridine as catalyst, dichloroethane as solvent, nitrogen protection mode, 60°C Stir for 12 hours, TLC tracking reaction, after the reaction is fully completed, column chromatography separation to obtain α-trifluoromethylthio substituted acetophenone products, the yield is between 61-85%, its reaction formula is as follows (1):
[0062]
[0063] Wherein, the proportion of aryl methyl ketone, sodium trifluoromethanesulfinate and triphosgene is 1:1.5:1 according to the molar concentration.
[0064] The outstanding innovation of the method of the present invention is that only cheap and green triphosgene is used as a reaction additive and pyridine is used as a catalyst, avoiding the use of trans...
Embodiment 1
[0066] The preparation method of α-trifluoromethylthioacetophenone, its synthetic route is as follows formula (3), carries out according to the following steps:
[0067]
[0068] (1) Add 1.0 mmol of acetophenone, 1.5 mmol of sodium trifluoromethanesulfinate, 1.0 mmol of triphosgene, and 0.1 mmol of pyridine into 3 mL of DCE, under nitrogen protection, and react at 60° C. for 12 hours.
[0069] (2) After the reaction was completed, 5 mL of 5% HCl aqueous solution was added to quench the reaction. Extracted twice with ethyl acetate (10 mL×2), washed once with saturated brine, and evaporated under reduced pressure to remove ethyl acetate. The oily target product was obtained by column chromatography with a yield of 82%.
[0070] The NMR data are as follows:
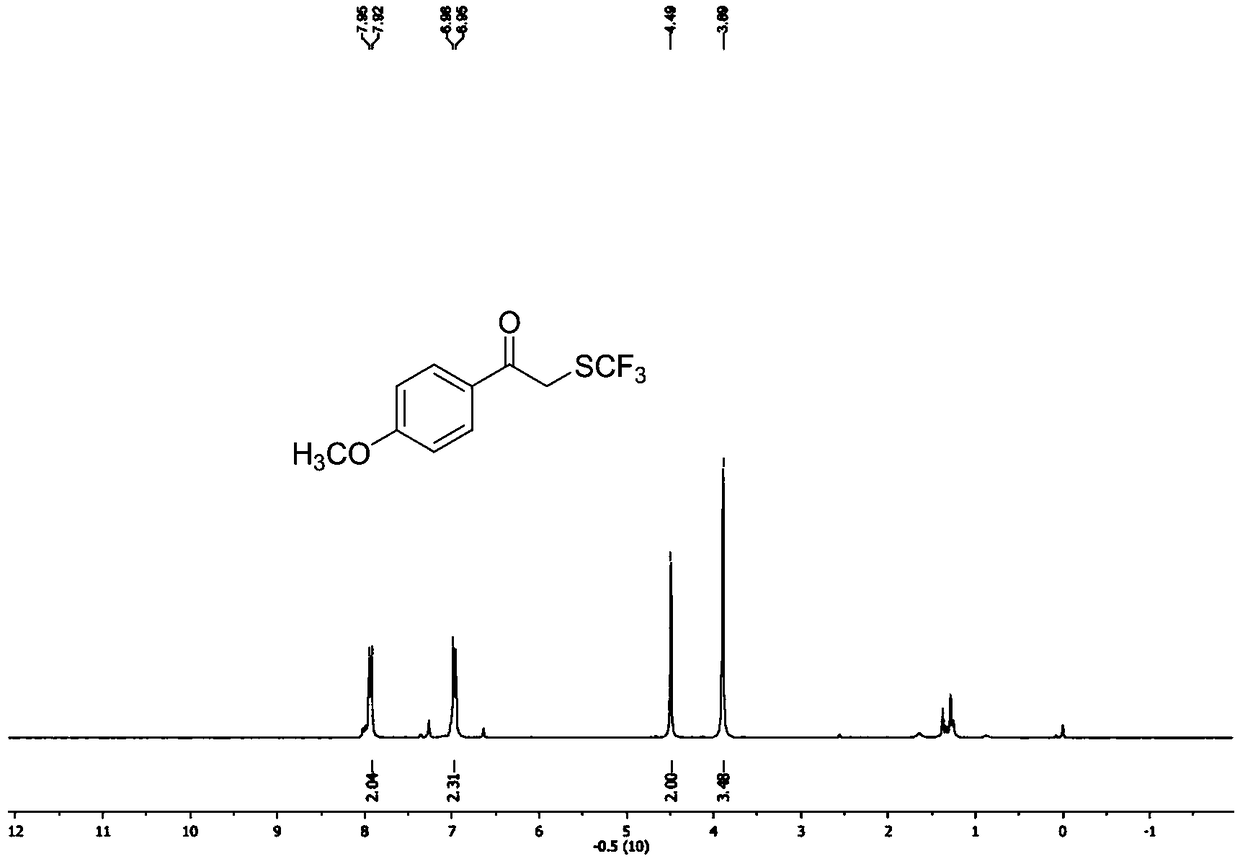
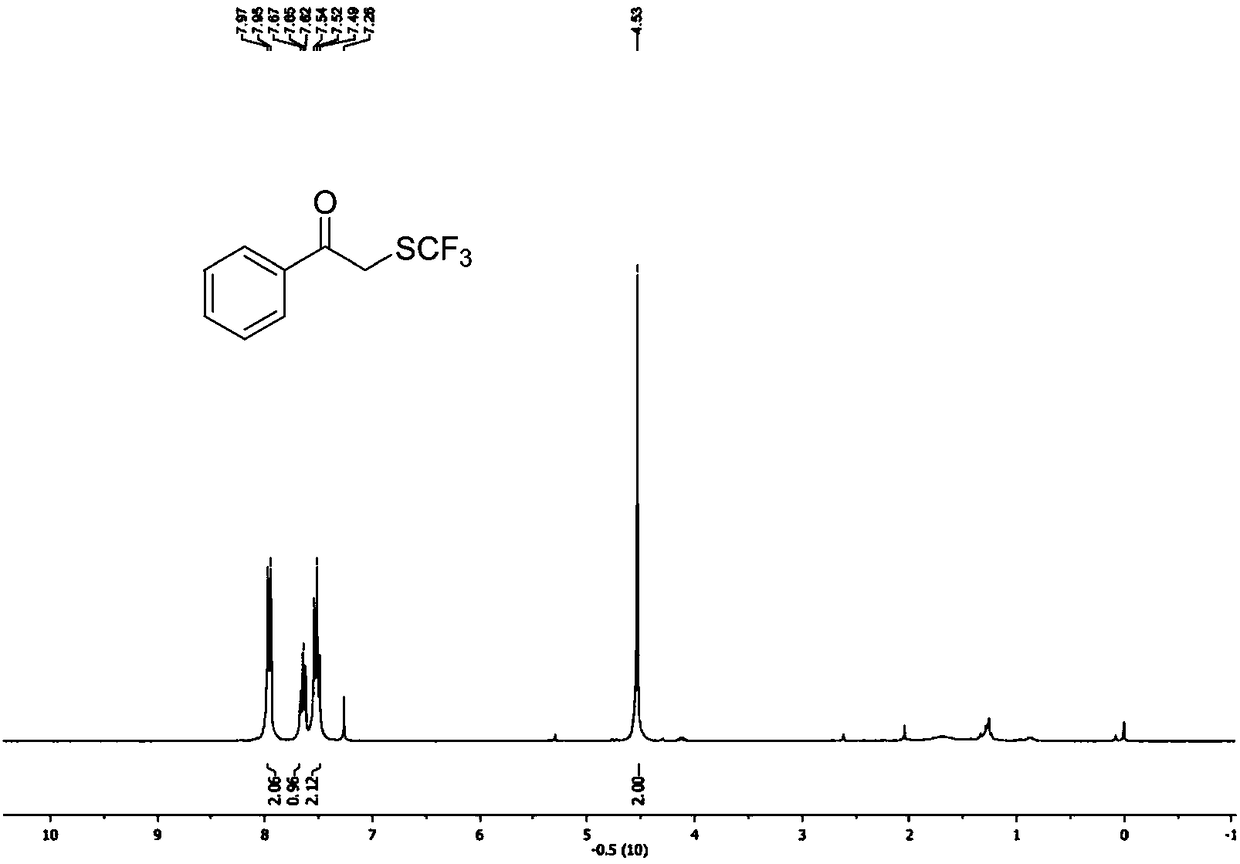
[0071] Such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3 ):δ=8.08-7.91(m,2H),7.67(t,J=7.3Hz,1H),7.53(t,J=7.9Hz,2H),4.54(s,2H).
[0072] Such as figure 2 as shown, 13 C NMR (101MHz, CDCl 3 ): δ=192.0(s), 134.7(s...
Embodiment 2
[0076] The preparation method of α-trifluoromethylthio-4-methylacetophenone, its synthetic route is as follows formula (4), carries out according to the following steps:
[0077]
[0078] (1) Add 1.0mmol of 4-methylacetophenone, 1.5mmol of sodium trifluoromethanesulfinate, 1.0mmol of triphosgene, and 0.1mmol of pyridine into 3mL of DCE, under nitrogen protection, and react at 60°C for 12 hours .
[0079] (2) After the reaction was completed, 5 mL of 5% HCl aqueous solution was added to quench the reaction. Extracted twice with ethyl acetate (10 mL×2), washed once with saturated brine, and evaporated under reduced pressure to remove ethyl acetate. The oily target product was obtained by column chromatography with a yield of 83%.
[0080] The NMR data are as follows:
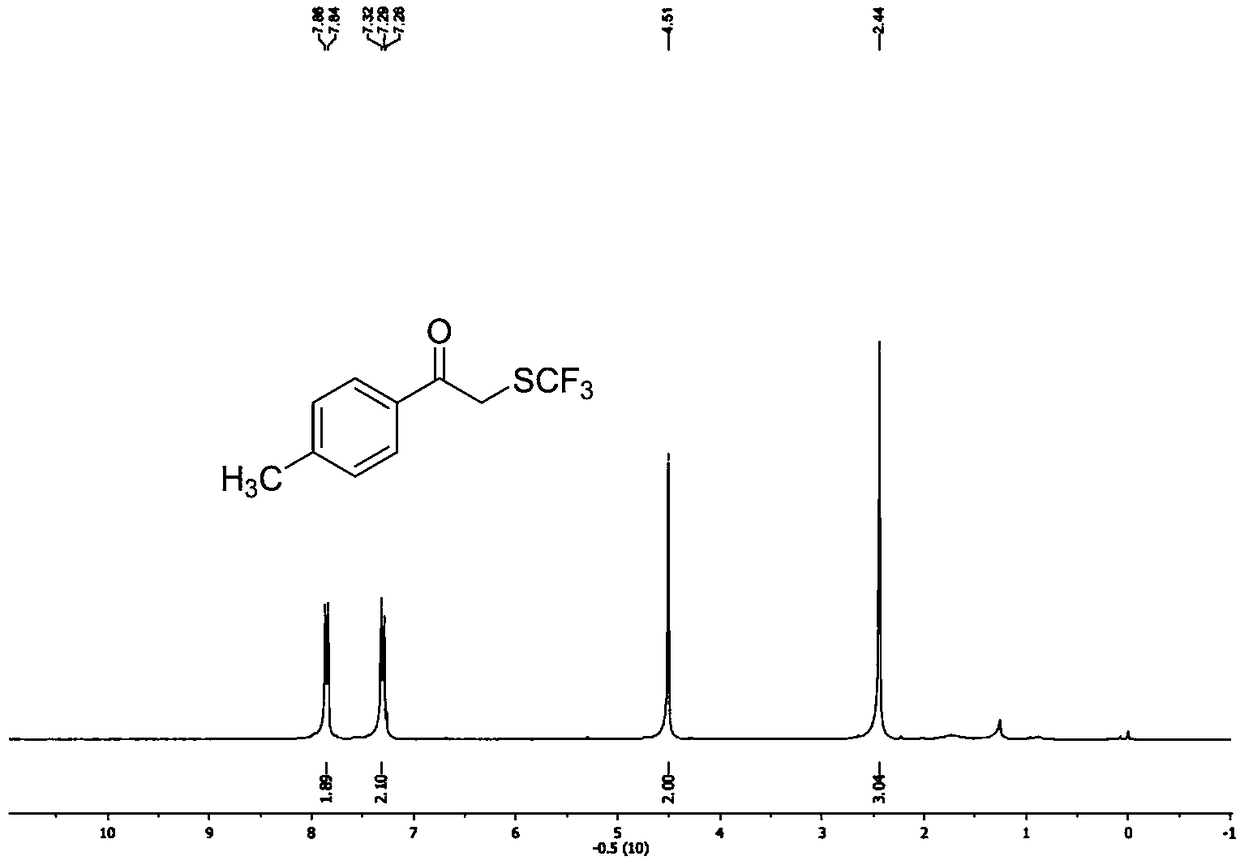
[0081] Such as image 3 shown, 1 H NMR (400MHz, CDCl 3 ):δ=7.87(d, J=8.2Hz, 2H), 7.33(d, J=8.2Hz, 2H), 4.52(s, 2H), 2.46(s, 3H).
[0082] Such as Figure 4 shown, 13 C NMR (101MHz, CDCl 3 ):δ=191.6(s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com