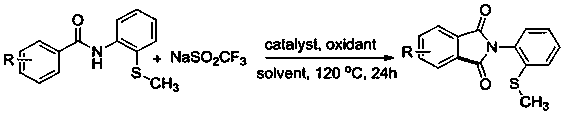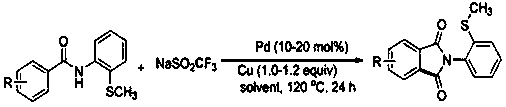Preparation method of N-(2-methylthiophenyl)isoindole-1,3-diketone compound
A technology of methylthiophenyl and compound, which is applied in the field of preparing N-isoindole-1,3-dione compound, can solve the problems of metal palladium catalyst poisoning, toxicity, and difficult synthesis of compounds, and achieve easy-to-obtain, The effect of simple operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
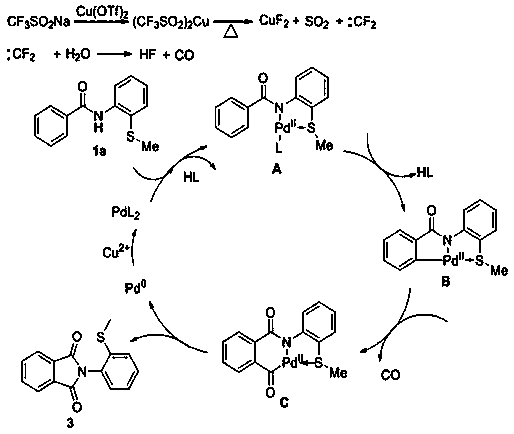
[0025] The invention discloses a N- (2-methylthiophenyl) the preparation method of isoindole-1,3-dione derivative, comprises the following steps: with N -(2-Methylthiophenyl)benzamide and sodium trifluoromethanesulfinate are the reaction substrates, and the molar ratio of the two is 1:2~1:3. By adding 10-20 mol% of palladium trifluoroacetate or palladium acetate as a catalyst, 100%-120 mol% of copper trifluoromethanesulfonate or copper tetrafluoroborate as one of the oxidants, in chlorobenzene or 1, 2-dichloroethane in one of the reaction solvents, reacted at a temperature of 120°C for 24 h; the chemical reaction formula is as follows:
[0026]
[0027] The -R is hydrogen, 2-methyl, 4-methyl, 3-methyl, 3,5-dimethyl, 4-ethyl, 4-methoxy, 3-methoxy, 3,4 -Methylenedioxy, 4-fluoro, 3-fluoro, 4-chloro, 3-chloro, 4-bromo, 4-iodo, 4-trifluoromethoxy, 4-trifluoromethyl, 4-phenyl , one of 2-naphthyl,
[0028] After the reaction is completed, after cooling, the reaction solution i...
specific Embodiment 1
[0029] Specific embodiment one: 48.6 milligrams (0.2 mmol) N -(2-Methylthiophenyl)benzamide, 87 mg (0.54 mmol) sodium trifluoromethanesulfinate, 17 mg (0.04 mmol) palladium trifluoroacetate, 86.7 mg (0.24 mmol) trifluoromethanesulfonic acid Copper, add 2 mL of chlorobenzene solvent. Reacted at room temperature at 120°C for 24 hours, cooled after the reaction, filtered, and the filtrate was rotary evaporated to remove the solvent, and the residue was subjected to silica gel column chromatography, rinsed with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 10:1, The effluent was collected according to the actual gradient, detected by TLC, and the effluent containing the product was combined, and the solvent was distilled off by a rotary evaporator, and dried in vacuo to obtain 41.4 mg of a white solid. - (2-Methylthiophenyl)isoindole-1, 3-dione, yield 77%. White solid. m.p. 158-160 o c. 1 H NMR (500 MHz, CDCl 3) δ 7.97 - 7.96 (m, 2H), 7.81 - 7.7...
specific Embodiment 2
[0030] Specific embodiment two: 51.4 milligrams (0.2 mmol) 2-methyl- N -(2-Methylthiophenyl)benzamide, 87 mg (0.54 mmol) sodium trifluoromethanesulfinate, 17 mg (0.04 mmol) palladium trifluoroacetate, 86.7 mg (0.24 mmol) trifluoromethanesulfonic acid Copper, add 2 mL of chlorobenzene solvent. Reacted at room temperature at 120°C for 24 hours, cooled after the reaction, filtered, and the filtrate was rotary evaporated to remove the solvent, and the residue was subjected to silica gel column chromatography, rinsed with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 10:1, The effluent was collected according to the actual gradient, detected by TLC, the effluent containing the product was combined, and the solvent was distilled off by a rotary evaporator, and the residue was chromatographed on a silica gel column, washed with a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 10:1 , collect the effluent according to the actu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com