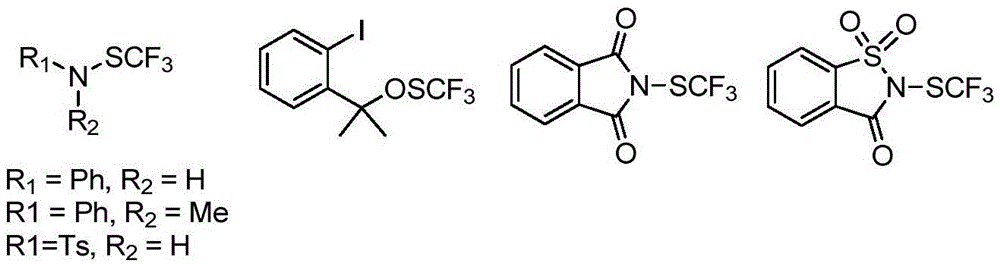
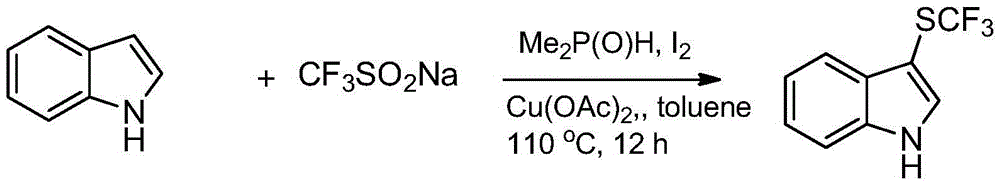Trifluoromethylthiolation reagent and application thereof
A technology of trifluoromethylthio and sodium trifluoromethylsulfinate, applied in the direction of organic chemistry, etc., can solve the problems of high toxicity, difficulty in preparation and expensive raw materials of trifluoromethylthio flower reagent, and achieve high yield , Substrate adaptability, cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a reactor containing solvent toluene, add sodium trifluoromethylsulfinate (62.4g), indole (23.4g), dimethyl phosphite (55mL), iodine element (about 10g) and copper acetate (50g ), stirred at 110° C. for more than 12 hours under nitrogen protection conditions. After the reaction, the product 3-trifluoromethylthioindole was obtained after filtration, distillation and column chromatography separation. , the yield was 89%.
Embodiment 2
[0023] In a reactor containing solvent toluene, add sodium trifluoromethanesulfinate (62.4g), indole (23.4g), diethyl phosphite (60mL), iodine element (about 10g) and copper acetate (50g ), stirred at 110° C. for more than 12 hours under nitrogen protection conditions. After the reaction, the product 3-trifluoromethylthioindole was obtained after filtration, distillation and column chromatography separation. , and the yield was 87%.
Embodiment 3
[0025] In the reactor containing solvent toluene, add sodium trifluoromethyl sulfinate (62.4g), N-methylindole (26.2g), diethyl phosphite (60mL), iodine simple substance (about 10g) and Copper acetate (50 g), stirred at 110° C. under nitrogen protection for more than 12 hours. After the reaction, the product N-methyl-3-trifluoromethylthioindole was obtained after filtration, distillation and column chromatography separation. , and the yield was 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com