Quinone-catalyzed trifluoromethylation photocatalytic synthesis method
A technology of trifluoromethylated photo-synthesis method, which is applied in the field of photocatalytic organic synthesis, can solve the problems of high pollution and high cost, achieve low environmental pollution, overcome consumption, and be beneficial to large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Mix monocyclic quinones (different quinones) and sodium trifluoromethanesulfinate with a molar ratio of 100:200; take the mixed sample in a manganese dioxide fixed-bed reactor, and then mix 15ml of acetonitrile and 10ml of benzene Pour in the reactor, under air atmosphere, 800mW visible light irradiation 12h, obtain the product liquid of uniform transparency; Figure 4 Shown; The mass spectrometry and nuclear magnetic resonance spectra of the product liquid are as figure 1 , 2 shown).
Embodiment 2
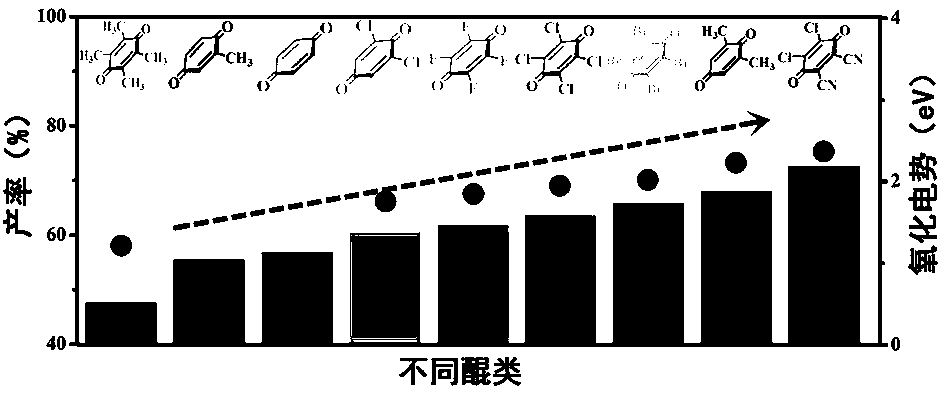
[0033] Mix benzoquinone and sodium trifluoromethyl sulfinate with a molar ratio of 100:200; take the mixed sample in a manganese dioxide fixed-bed reactor, then pour 15ml of acetonitrile and 10ml of benzene into the reactor, in an air atmosphere Under the condition of light intensity ranging from 200-800mW, visible light ranging from light wavelength 420-780nm was irradiated for 10h to obtain a uniform and transparent product liquid. (it records reaction light intensity by light meter, records the ultraviolet absorption diffuse reflectance spectrum of quinone catalyst by DRS. The relation of reaction light intensity, reaction wavelength and product yield is as follows Figure 5 Shown; The mass spectrometry and nuclear magnetic resonance spectra of the product liquid are as figure 1 , 2 shown).
Embodiment 3
[0035] Mix monocyclic quinones and sodium trifluoromethanesulfinate with a molar ratio of 100:200; take the mixed sample in a manganese dioxide fixed-bed reactor, then mix 15ml of acetonitrile and 10ml of 5,6-dimethoxy Base-1-indanone (Aricept drug precursor) was poured into the reactor, and 800mW of visible light of different wavelengths was irradiated for 15 hours under an air atmosphere to obtain a uniform and transparent product liquid. (mass spectrum, nuclear magnetic resonance spectrum of product liquid such as Figure 6 , 7 shown).
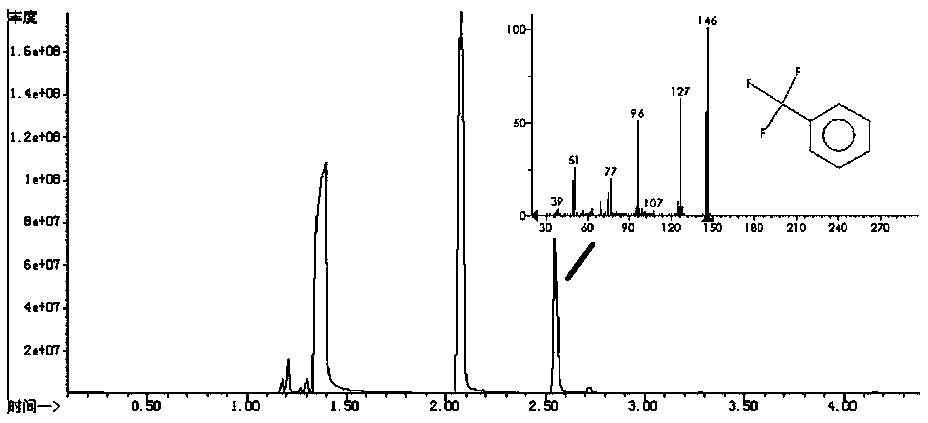
[0036] The GC-MS spectrogram of the trifluorotoluene that the present invention reacts obtains ( figure 1 ) The peak at 2.5 min was matched to trifluorotoluene. After repeating the experiment, by nuclear magnetic resonance, 19 F spectrum ( figure 2 ) Analysis 62.68ppm place is the standard trifluorotoluene peak. Trifluoromethylation reaction with 5,6-dimethoxy-1-indanone (Aricept drug precursor) as substrate, we obtained a variety of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com