Preparation method of oxidation impurity
A technology for oxidizing impurities and oxidation reactions, applied in organic chemistry, steroids, etc., and can solve problems such as no relevant literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
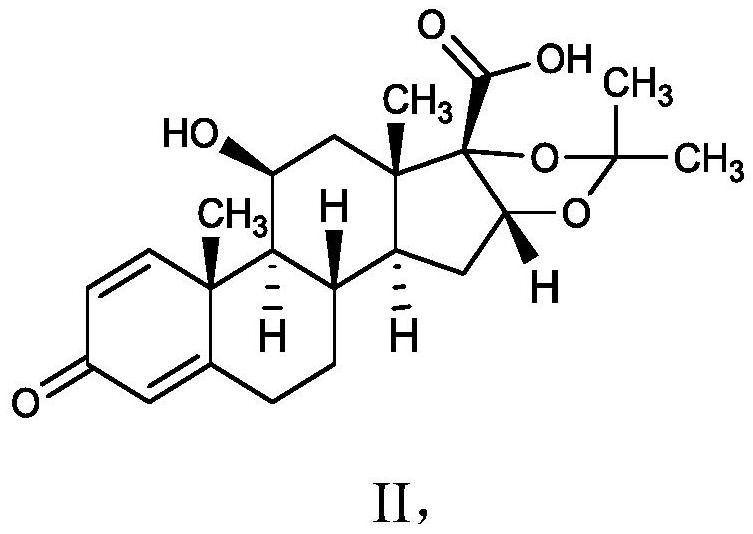
[0045] Example 1 Preparation of 11β, 16α, 17α-trihydroxy-3-oxoandrost-1,4-diene-17-carboxylic acid ring 16,17-acetal acetone
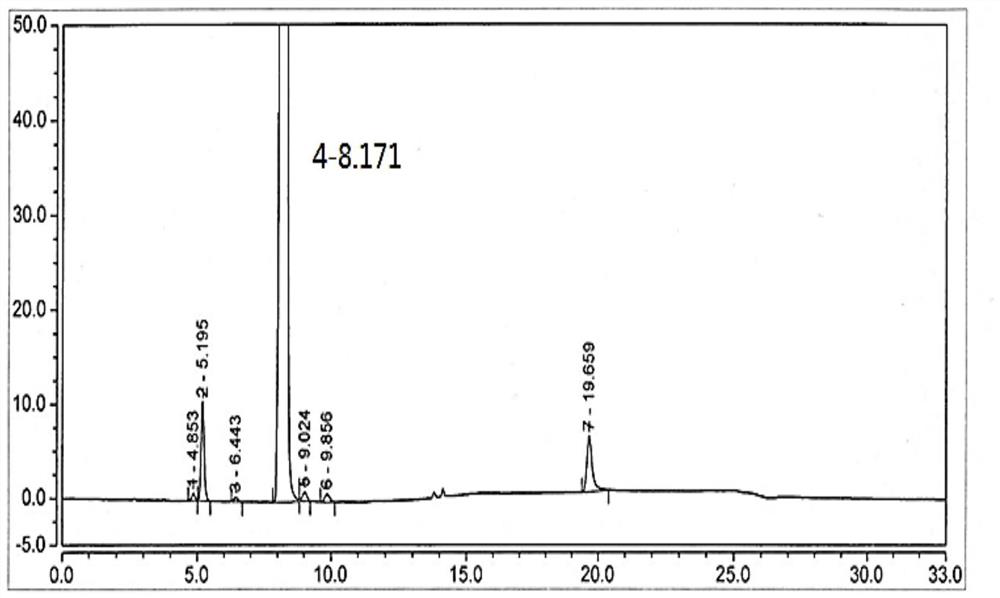
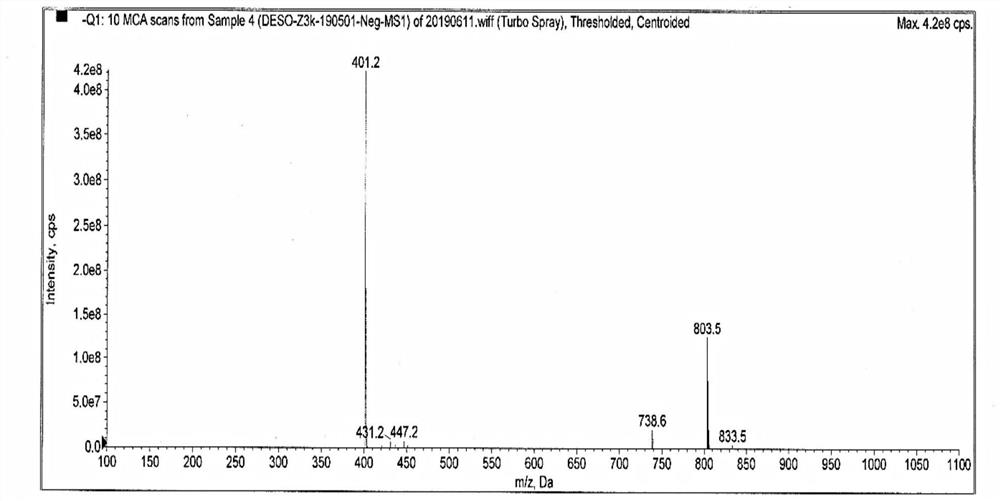
[0046] Add 7.5g of desonide, 2300ml of tetrahydrofuran, 200ml of water, 5g of potassium hydroxide into a 3000ml three-neck flask, and let air flow in at 20-25°C for 16 hours. TLC spotting (CH2Cl2:CH3OH15:1) shows that the reaction of the raw materials is complete, stop To react, add 700ml of saturated brine / 500ml of ethyl acetate to separate layers, and concentrate the organic layer to dryness; add 700ml of ethyl acetate and 230ml of water, adjust the pH value to 2.5-3.5 with hydrochloric acid, separate the layers, and dry the organic layer with anhydrous sodium sulfate for 2 hours. Concentrate to dryness at 40-50°C, then dry under reduced pressure at 40-50°C for 4h to obtain 4.3g of solid, with HPLC purity of 98.2% ( figure 1 ), the integration results are shown in the table below, and the mass spectrum is shown in figure 2 .
[0047]
Embodiment 2
[0048] Example 2 Preparation of 11β, 16α, 17α-trihydroxy-3-oxoandrost-1,4-diene-17-carboxylic acid ring 16,17-acetal acetone
[0049] Add 7.5g of desonide, 2300ml of methanol, 200ml of water, and 3g of sodium hydroxide into a 3000ml three-necked flask, and feed air at 30-45°C for 19 hours. TLC spotting (CH2Cl2:CH3OH 15:1) shows that the reaction of the raw materials is complete. Stop the reaction, add 700ml saturated brine / 500ml ethyl acetate to separate layers, concentrate the organic layer to dryness; add 700ml ethyl acetate and 230ml water, adjust the pH value to 2.5-3.5 with hydrochloric acid, separate the layers, and dry the organic layer with anhydrous sodium sulfate for 2 hours , concentrated to dryness at 40-50° C., and then dried under reduced pressure at 40-50° C. for 4 hours to obtain 4.5 g of solid with an HPLC purity of 97.62%.
Embodiment 3
[0050] Example 3 Preparation of 11β, 16α, 17α-trihydroxy-3-oxoandrost-1,4-diene-17-carboxylic acid ring 16,17-acetal acetone
[0051] Add 7.5g of desonide, 2300ml of ethanol, 200ml of water, and 6g of lithium hydroxide into a 3000ml three-neck flask, and let air flow in at 10-15°C for 13 hours. TLC spotting (CH2Cl2:CH3OH 15:1) shows that the reaction of the raw materials is complete. Stop the reaction, add 700ml saturated brine / 500ml ethyl acetate to separate layers, concentrate the organic layer to dryness; add 700ml ethyl acetate and 230ml water, adjust the pH value to 2.5-3.5 with hydrochloric acid, separate the layers, and dry the organic layer with anhydrous sodium sulfate for 2 hours , concentrated to dryness at 40-50° C., and then dried under reduced pressure at 40-50° C. for 4 hours to obtain 3.8 g of solid, with an HPLC purity of 97.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com