A kind of preparation method of budesonide sterile raw material and its suspension for inhalation
A technology for suspension and raw materials, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of unstable budesonide aseptic raw materials and low sterility assurance. , to achieve the effect of high sterility guarantee and good sterilization effect of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A kind of preparation method of budesonide suspension provided by the invention comprises:
[0041] Step 1: Prepare a suspension: disperse the sterile raw material of the water-insoluble drug budesonide in the sterilized auxiliary material solution, and the dispersion speed is 2000-20000 rpm to obtain a suspension containing budesonide;
[0042] Step 2: High-pressure homogenization: The suspension containing budesonide in step 1 is wet-micronized by a high-pressure homogenizer to a suitable particle size distribution, and the budesonide suspension is obtained by constant volume with water for injection, wherein the high-pressure homogenization The qualitative pressure is 100~
[0043] 1500bar, high-pressure homogenization of the suspension until the particle size distribution is d(v, 0.5) = 1-3 μm, d(v, 0.5) < 5 μm;
[0044] Step 3: potting: potting the budesonide suspension obtained in step 2 into an ampoule to prepare a budesonide suspension for inhalation.
Embodiment 1
[0047] Comparison of physical and chemical properties of bulk drug budesonide before and after nitrogen filling sterilization
[0048] In this embodiment, a series of experiments were carried out to confirm the effects of sterilization temperature, time and nitrogen filling on the content of budesonide bulk drug and related substances.
[0049] The specific measures are as follows:
[0050]Sterilize in a hot air disinfection box, the model of the hot air disinfection box is BGZ-30, fill the raw material of budesonide with nitrogen, heat and sterilize the budesonide after filling with nitrogen, and the sterilization condition of 1# sample is 160 ° C × 190 min (filling with nitrogen) Nitrogen), the sterilization condition of 2# sample is 170°C×120min (nitrogen filled), the sterilization condition of 3# sample is 130°C×120min (not filled with nitrogen), the sterilization condition of 4# sample is 160°C×120min (not filled with nitrogen). After the sterilization treatment, the ch...
Embodiment 2
[0055] Comparison of physical and chemical properties of bulk drug budesonide before and after nitrogen filling sterilization
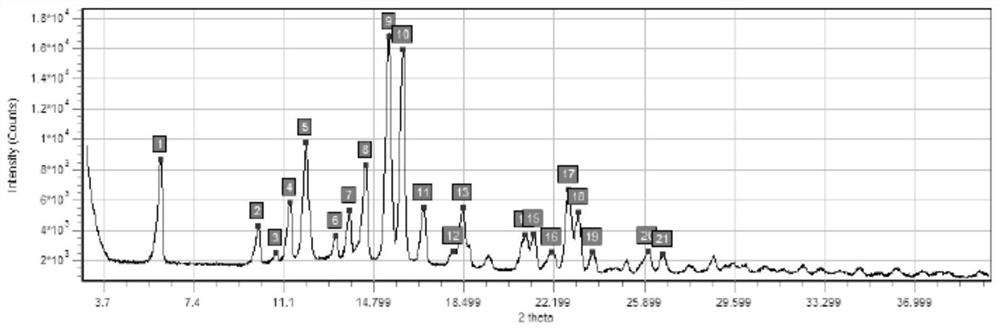
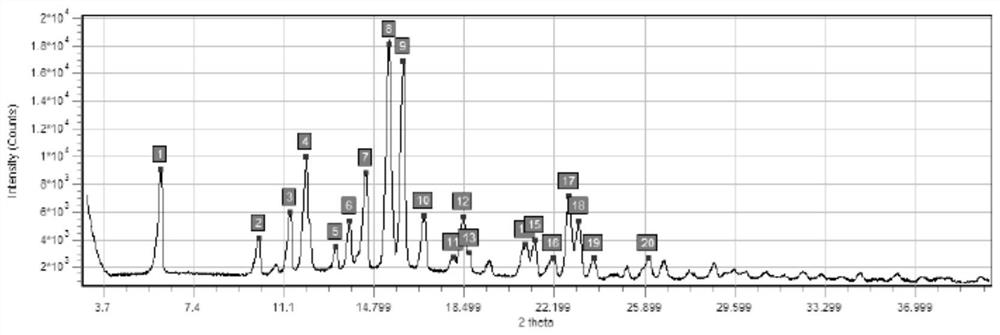
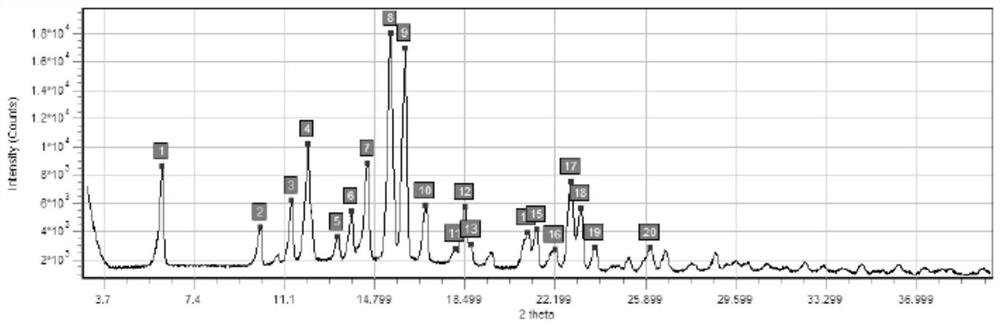
[0056] In this embodiment, a series of experiments were carried out to confirm the influence of sterilization temperature, time and nitrogen filling on the crystal form of budesonide bulk drug.
[0057] The specific measures are as follows:
[0058] Sterilize with a hot air disinfection box, the model of the hot air disinfection box is BGZ-30, fill the bulk drug of budesonide with nitrogen, heat and sterilize the budesonide after nitrogen filling, and the sterilization condition of sample 1 is 160 ° C × 190min (nitrogen filling ), the sterilization condition of sample 2 is 170°C×120min (nitrogen filling), see Table 2 below for details.
[0059] Table 2: Conditions for dry heat sterilization of Budesonide API
[0060] Numbering raw material 1# 2# temperature / ℃ / 160 170 time / min / 190 120 Whether to fill nitrogen / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com