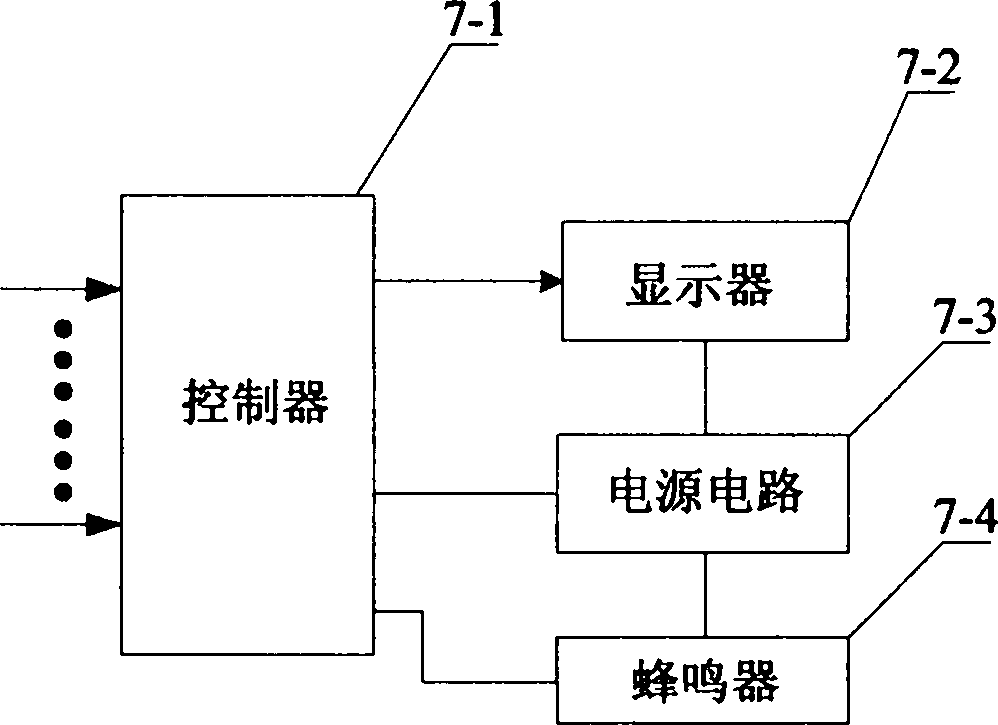
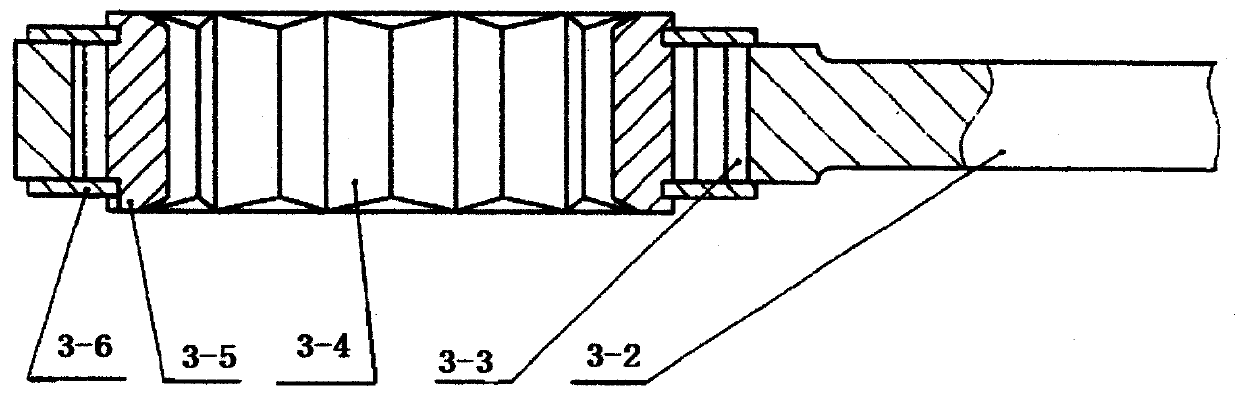
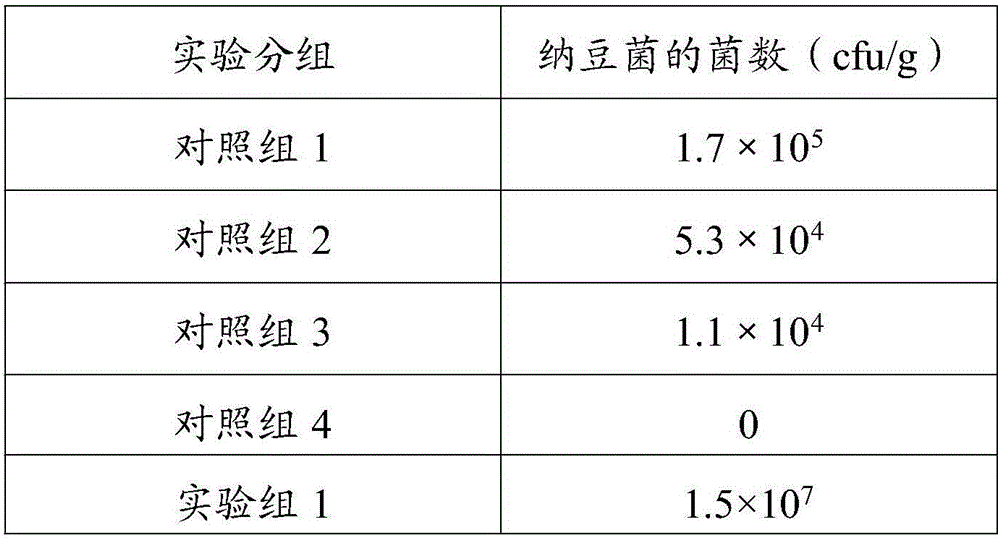
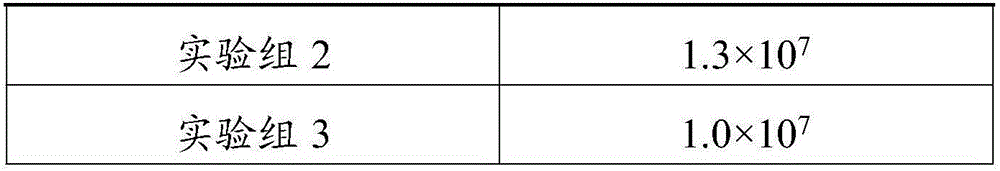
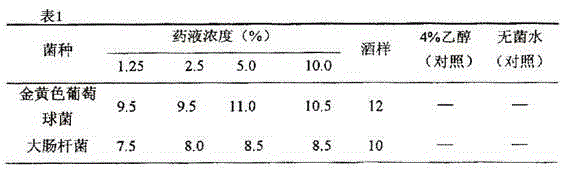
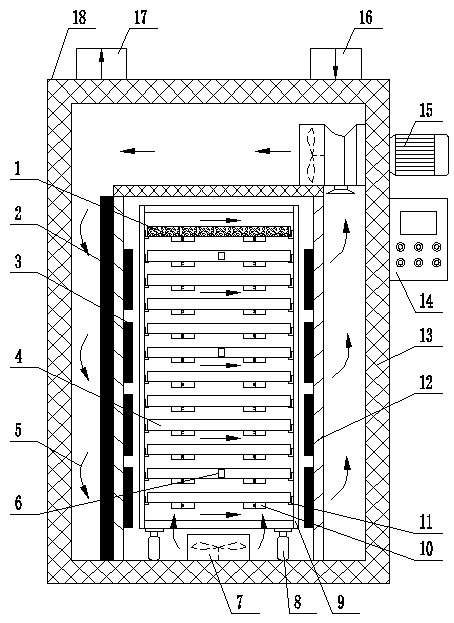
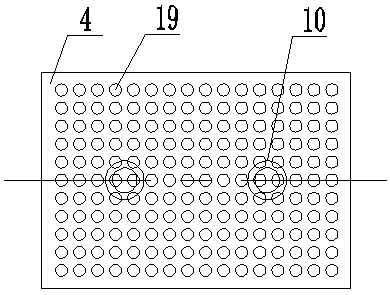
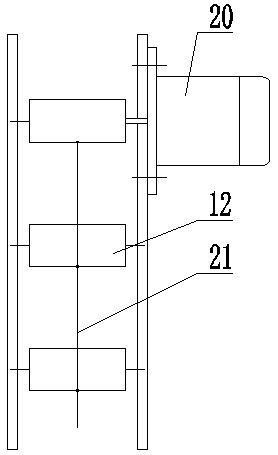
Patents
Literature
95 results about "Dry Heat Sterilization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
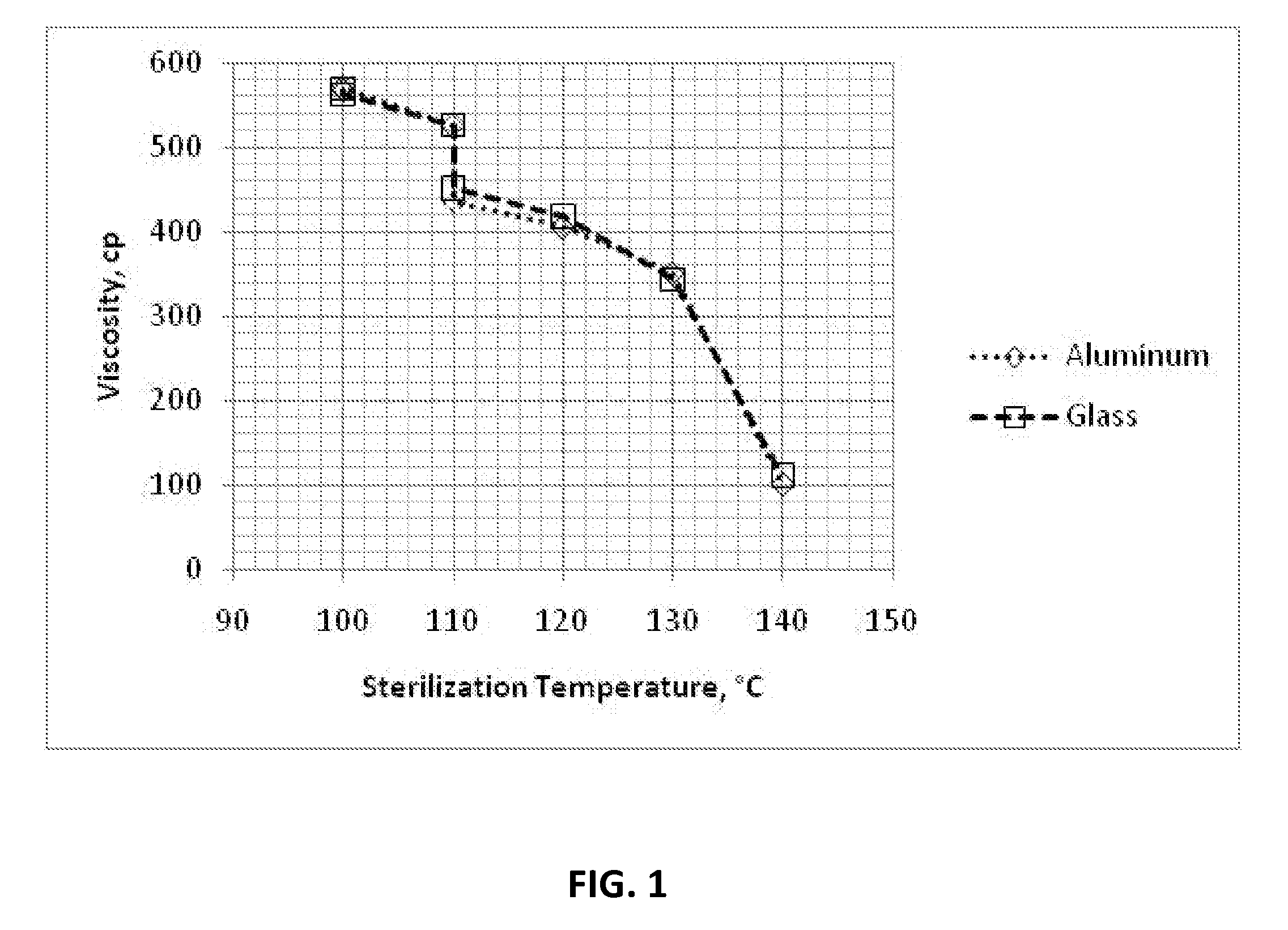
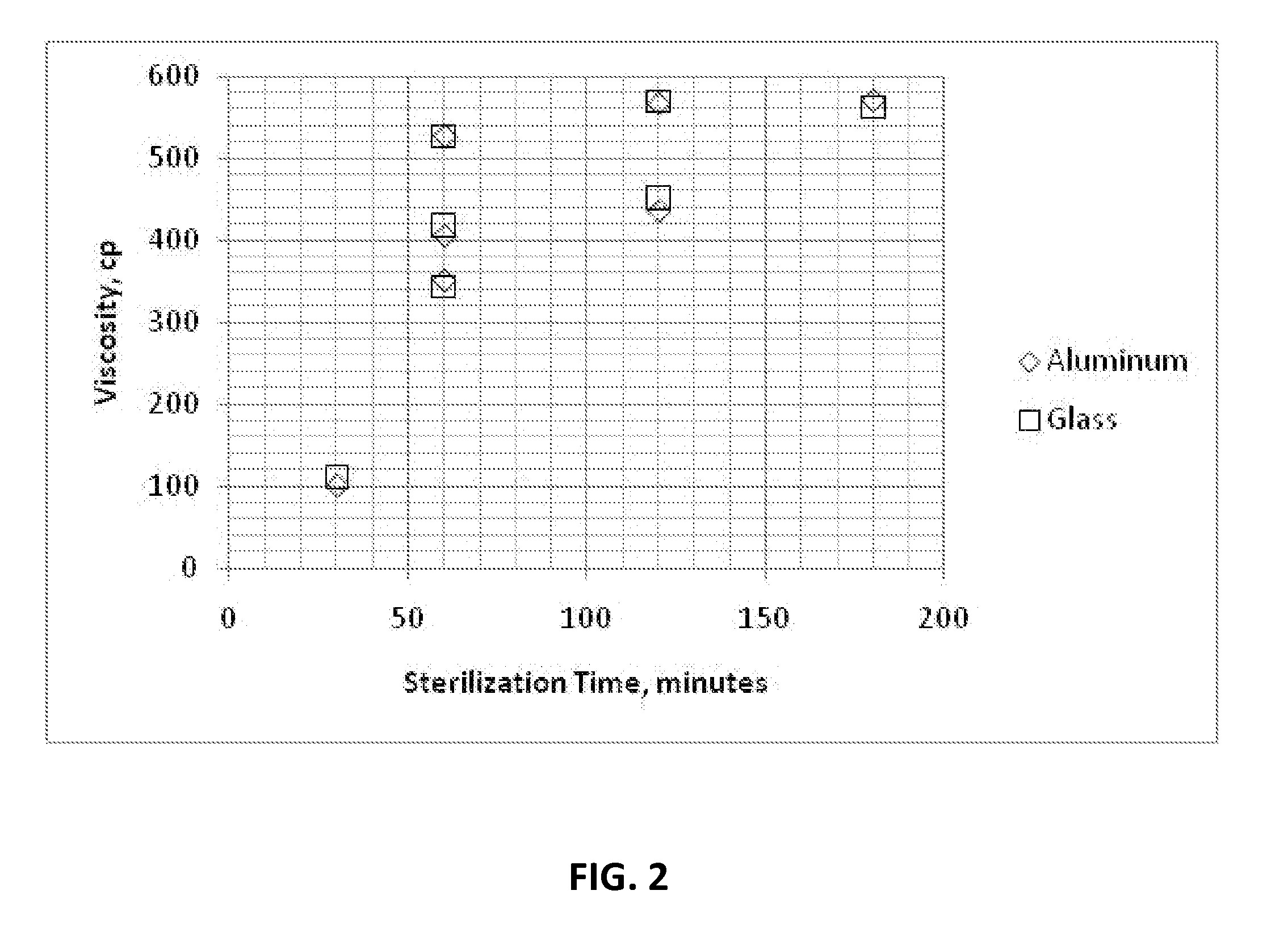
Dry heat sterilization of an article is one of the earliest forms of sterilization practiced. It uses hot air that is either free from water vapor or has very little of it, where this moisture plays a minimal or no role in the process of sterilization.
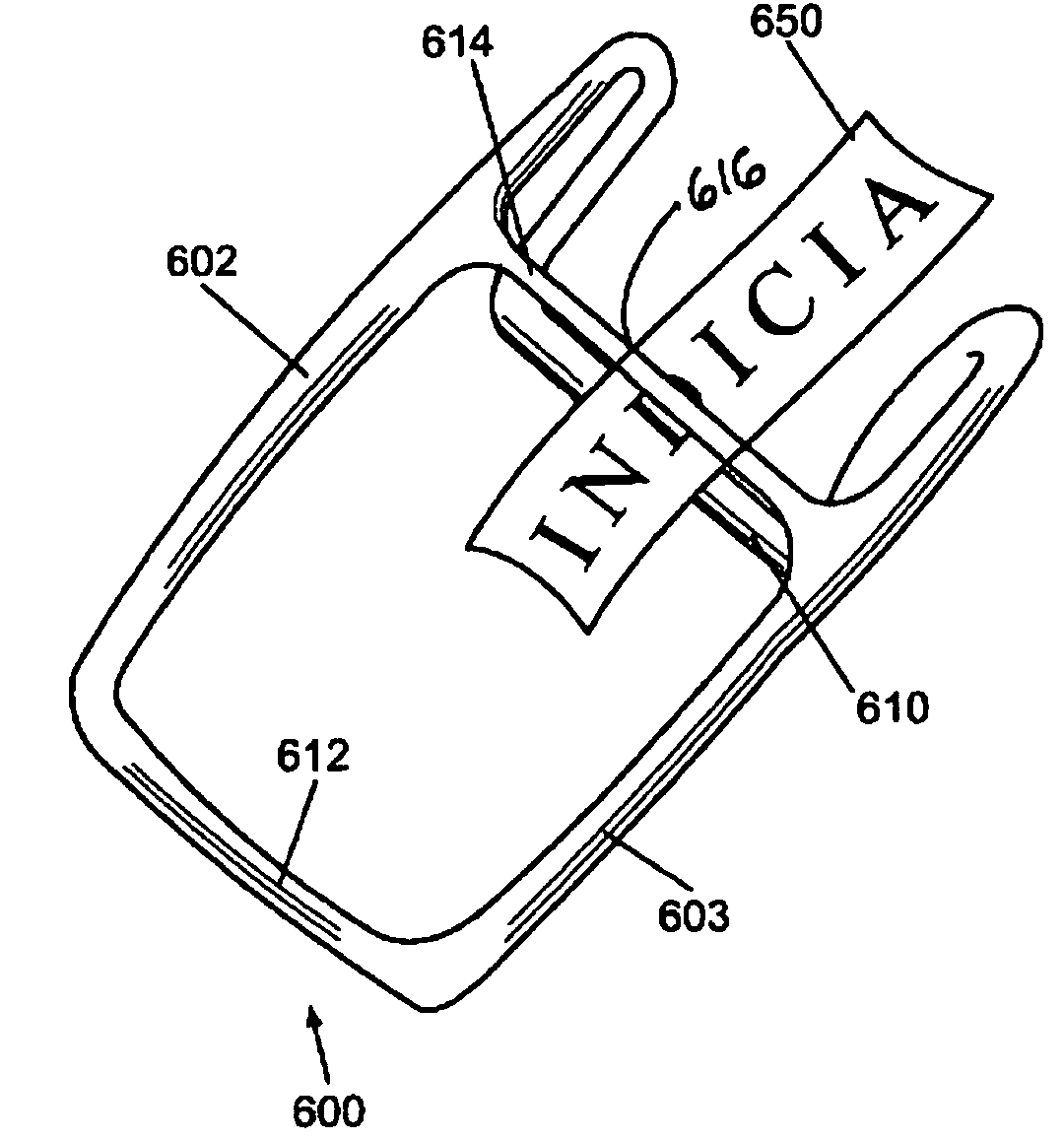
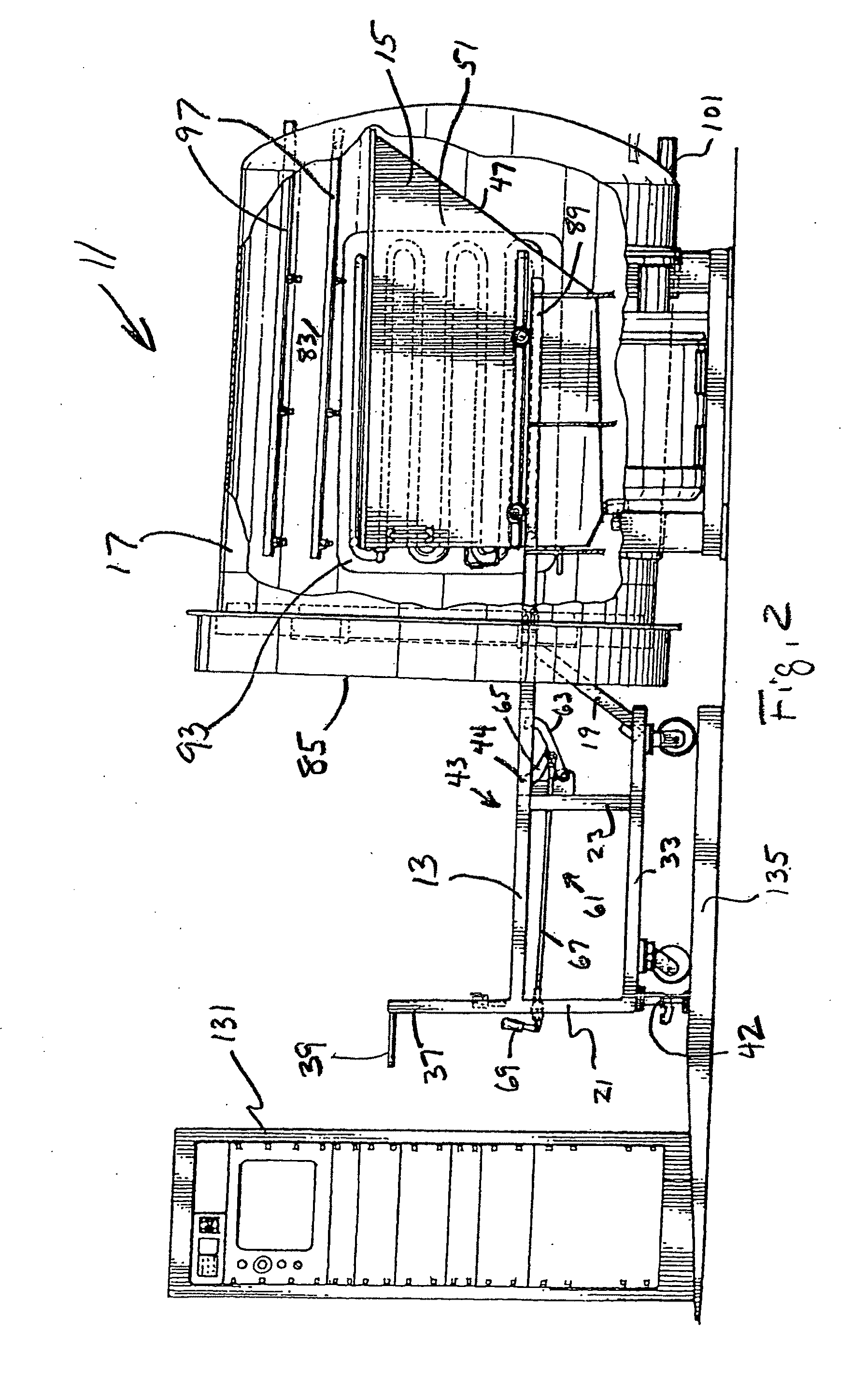
Disposable photographic cheek retraction apparatus and method of using the same
InactiveUS6988893B2Economically manufacturedReduce needSurgeryLip/mouth protectorsEngineeringHigh pressure
In an exemplary embodiment in accordance with the present invention, a disposable dental appliance and method of use is provided. In particular, a cheek retraction apparatus is provided, which is formed from a lightweight yet durable biocompatible polymer. The apparatus is sufficiently durable to withstand recurrent use, however, the it is economically manufactured so as to be disposable. Moreover, the apparatus is pre-sterilized to alleviate the need for autoclaving and / or dry heat sterilization. A cheek retraction apparatus in accordance with the present invention also provides an indicia display medium that allows the dental practitioner to display patient information, whitening treatment measurements, or other indicia that might be useful to them during oral photography.
Owner:MEDICAL COLLEGE OF GEORGIA RES INST
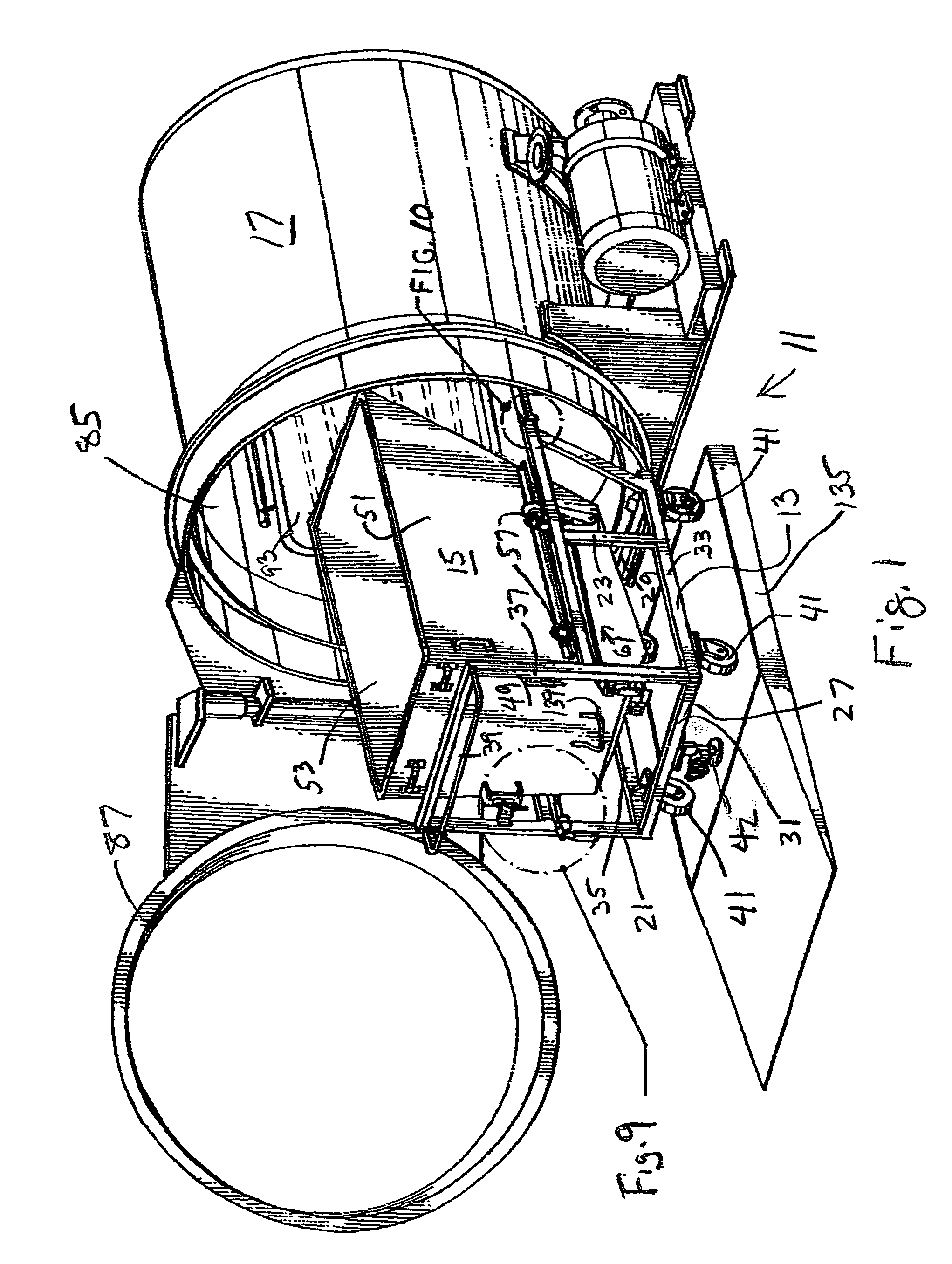
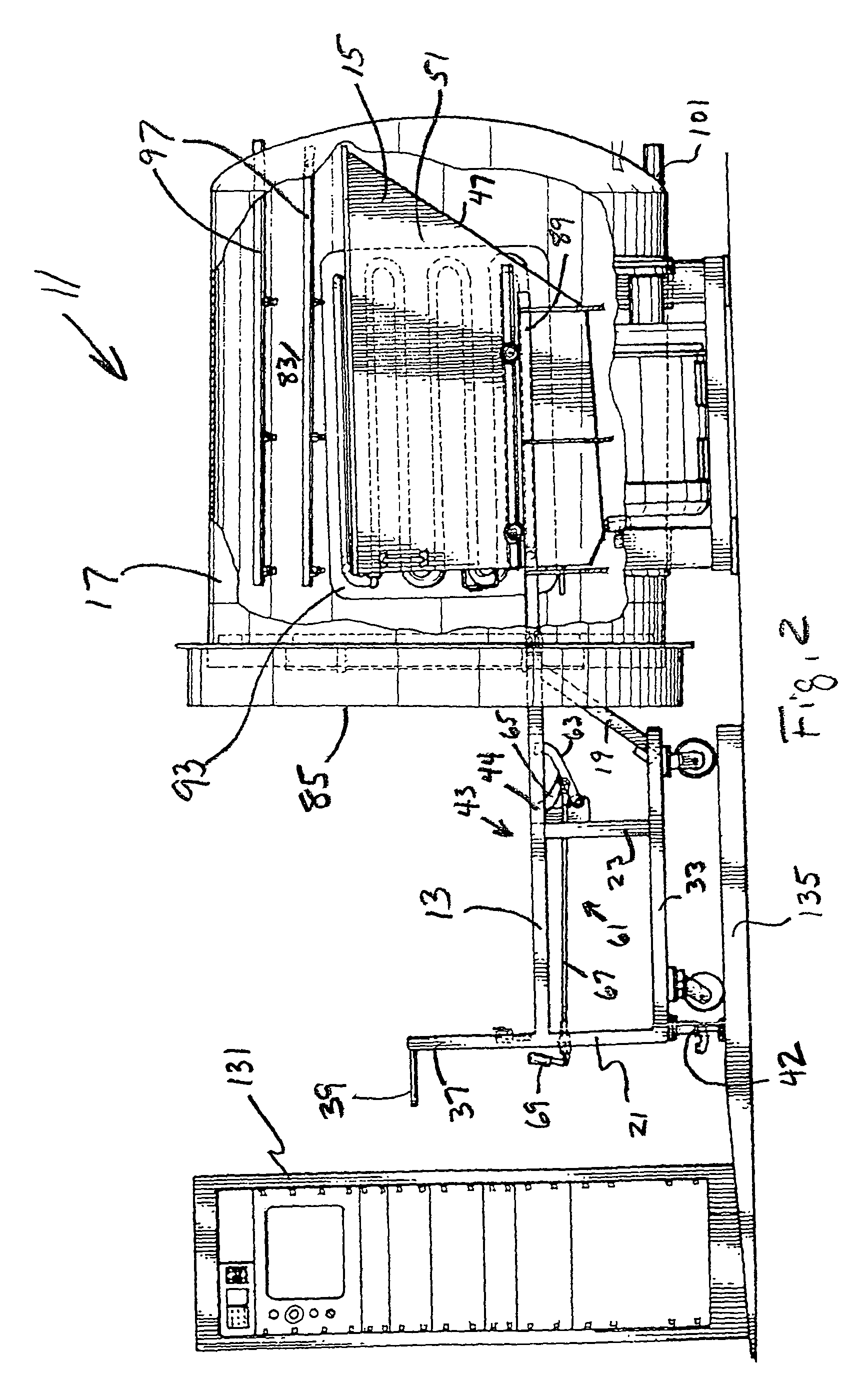
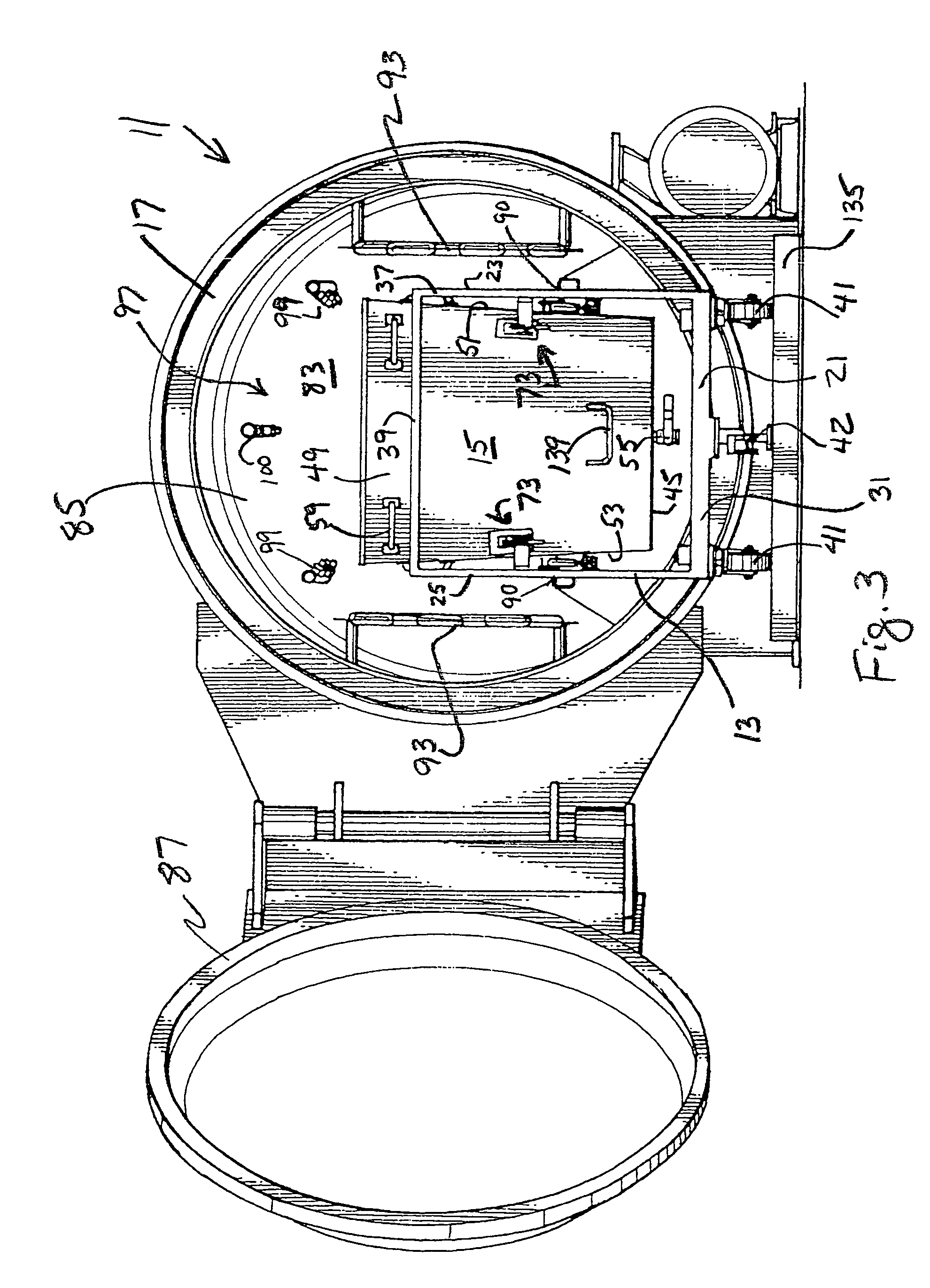
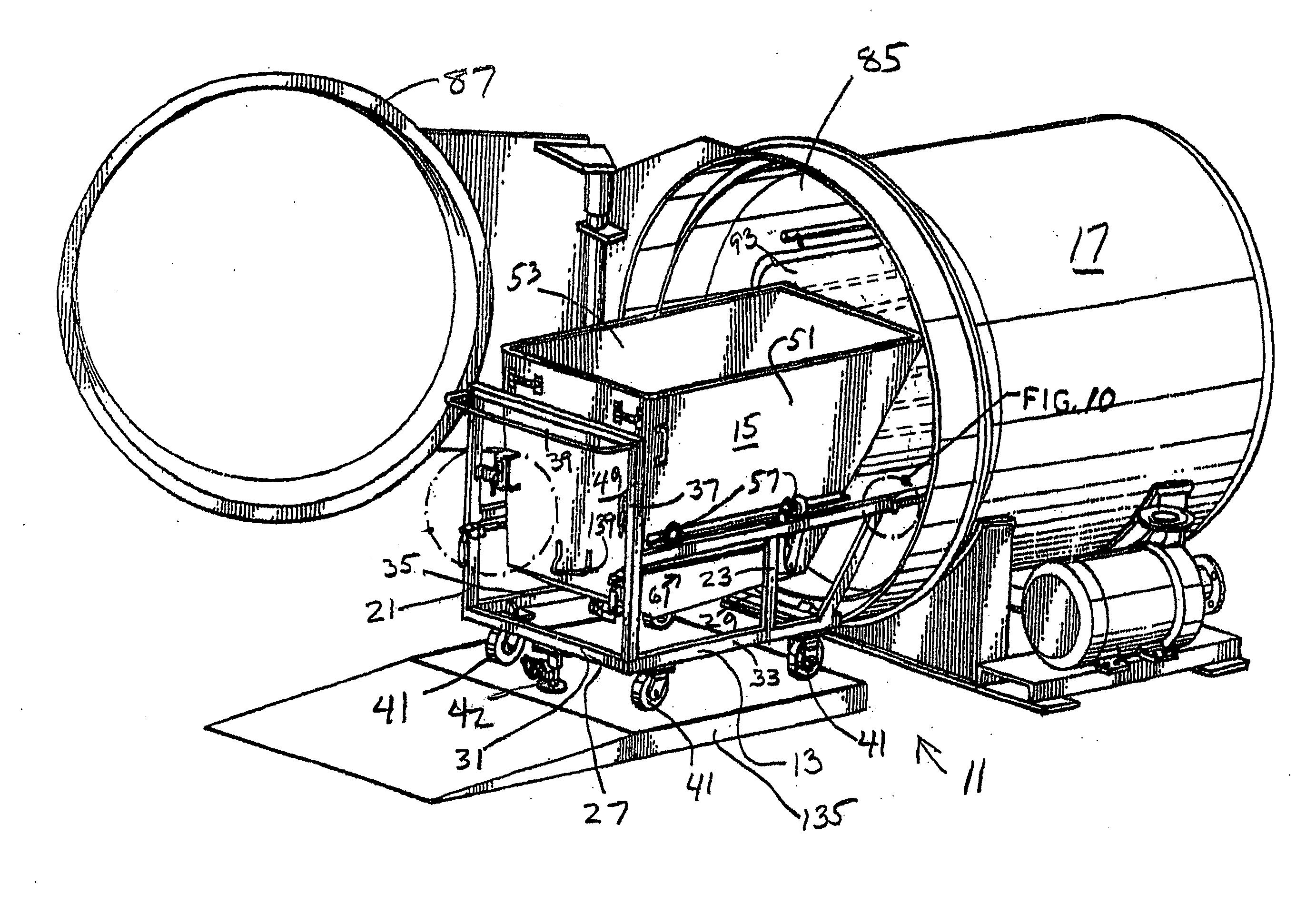
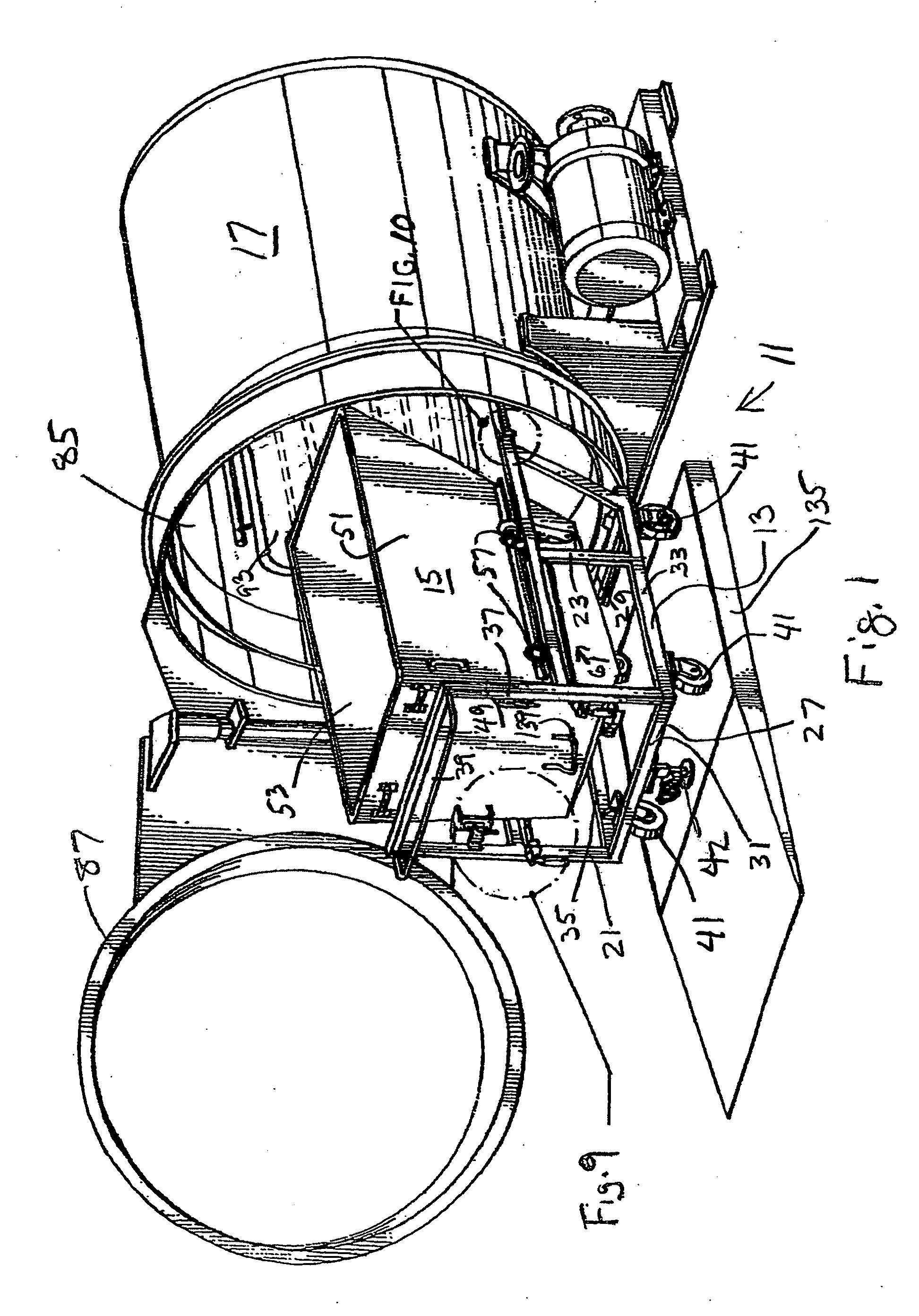
Steam sterilization system for sterilizing medical waste
InactiveUS7815851B1Reduce handlingReducing pathogen exposureLavatory sanitoryHeatThermal energyMedical waste
An integrated sterilization and materials handling system for sterilizing and handling items such as medical waste, paper, or other things comprises a cart for carrying a removable bin for holding the items to be sterilized, and a sterilization chamber that receives the bin when it is removed from the cart. The various embodiments include a bin that is dumpable with the cart using existing dumping apparatus, a bin that pivots on the cart to permit dumping of the items from the bin by tilting the bin, and a bin used primarily in a dry heat sterilization process of the invention when sterilizing items that are not to be discarded after sterilization to facilitate loading and unloading of the bin. The system has thermal energy booster plates mounted in the sterilization chamber for providing dry radiant heat to the chamber, and the system may vary process times, process pressures, and process temperatures to predetermine settings that correlate to the weight of the items to be sterilized.
Owner:ASSURED WASTE SOLUTIONS LLC
Dry Heat Sterilizing Systems Using Inductive Heating
InactiveUS20110211989A1Lavatory sanitoryEnergy based chemical/physical/physico-chemical processesEngineeringDry Heat Sterilization
A dry heat sterilizing system for use in sterilizing non-ferromagnetic and / or ferromagnetic parts includes a sterilization chamber and at least one ferromagnetic structure within the sterilization chamber. At least one inductive EMF energy field generator inductively heats the at least one ferromagnetic structure during a sterilization operation. The at least one ferromagnetic structure is in thermal communication with the non-ferromagnetic and / or ferromagnetic part during a sterilization operation to transfer heat from the ferromagnetic structure to the non-ferromagnetic and / or ferromagnetic part.
Owner:STATE OF FRANKLIN INNOVATIONS
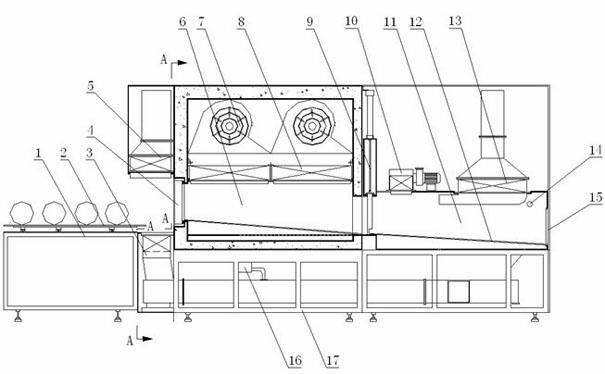
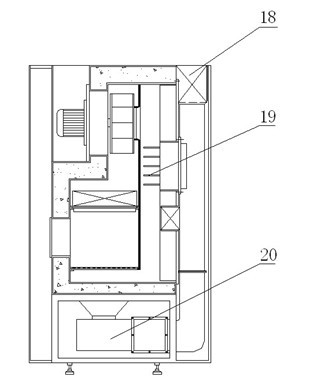
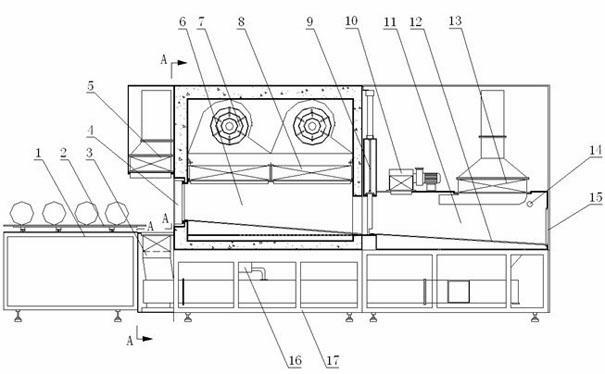
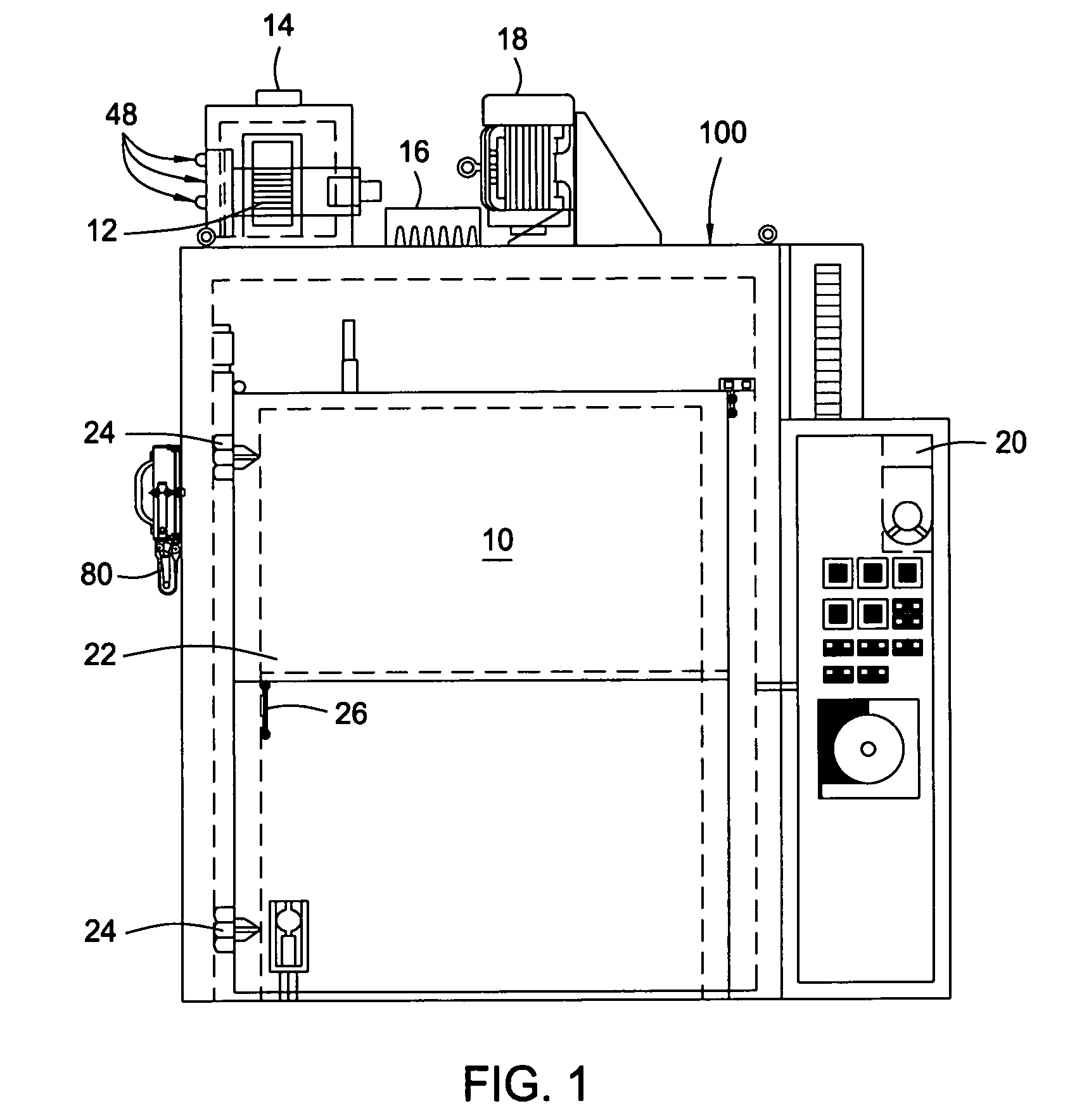
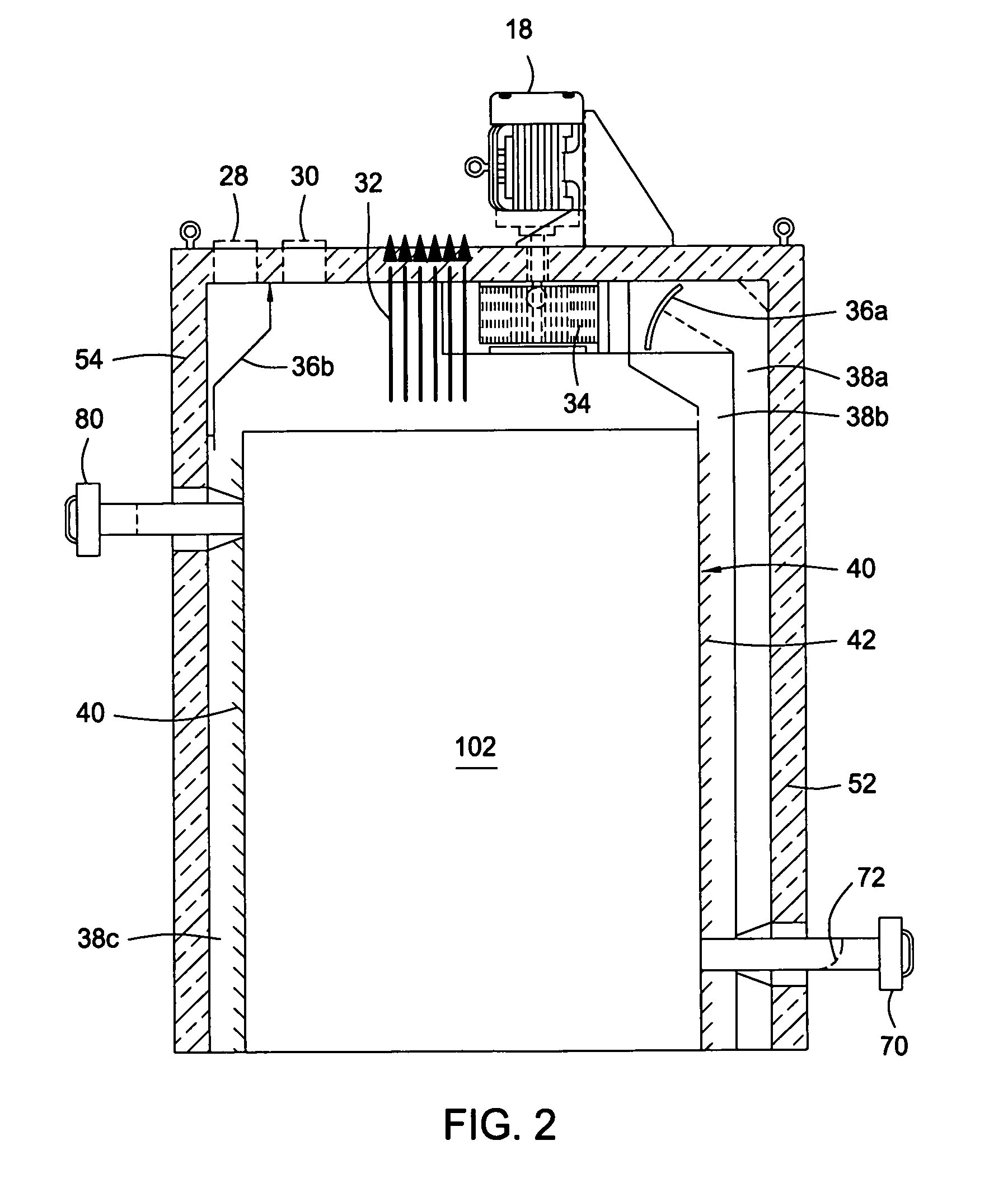
Dry heat convection sterilization system
InactiveUS20070237670A1Bacterial antigen ingredientsLavatory sanitoryAir blowerDry Heat Sterilization
A dry heat sterilization system having a housing and a number of plenums to guide the flow of hot air. Hot air is forced to flow through the plenums, the intake slide duct and into the bottom of the containers. The hot air will rise and exit from the top of the container through the exhaust slide duct. Furthermore, the external surfaces of the container are also sterilized through the use of semi-pierced duct walls with adjustable diffuser panels. Hot air will re-circulate until it reaches a pre-determined temperature. The container and its contents can be safely handled once the exhaust air blower removes hot air from the system.
Owner:TPS
Sterilization process design for a medical adhesive
ActiveUS8808620B1Effective sterilizationReduce the risk of infectionLavatory sanitoryHeatAdhesiveEngineering
Medical devices, including medical adhesives, need to be sterile before application to a patient. A dry heat sterilization process can sterilize medical adhesives for patient application. The dry heat sterilization process can be validated for particular equipment arrangements and medical adhesives being utilized.
Owner:TYCO HEALTHCARE GRP LP
Cyanoacrylate Adhesive Compositions and Devices and Process for Sterilization Thereof
The viscosity of 2-cyanoacrylate monomer based adhesives is increased by combining the monomers with suitable thickeners according to a certain process. The resulting formulations may be heat sterilized without degrading the viscosity or causing premature polymerization. The effectiveness of the sterilization process is assayed by disposing bacterial spores in the formulation, exposing it to a dry heat sterilization process, transferring it to a sterile aldose solution, transferring and exposing the sample to a nutrient medium which supports germination and growth of viable spores, incubating the samples, and determining the presence or absence of growth.
Owner:CLAST TRADING
Wheat bran medium for culturing fruit bat fly in laboratory
A wheat bran medium for culturing fruit bat fly in laboratory comprises 10g of wheat bran, 10 g of brown sugar, 1.2 g of agar, 1.2g of yeast and 0.15g of benzoic acid. A preparation method of the medium comprises steps of: dissolving the components in 100 ml of tap water and carrying out an autoclaving; taking glass test tube (25*200 mm) treated with dry heat sterilization (120 DEG C for 2h); subpackaging 20ml of medium into each test tube; cooling to a room temperature until the medium being solid gel; and putting fruit bat fly in the medium, wherein a test tube plug is absorbent cotton treated with dry heat sterilization. The easily prepared medium has low costs, good production effect and strong mildewing resistance, and is convenient for genetics and development biology researches on fruit bat fly.
Owner:许乐乐 +3
Method for high yield of organic tomatoes
InactiveCN105766308AImprove fertilityAchieve sustainable developmentPlant cultivationCultivating equipmentsOrganic farmingWater immersion
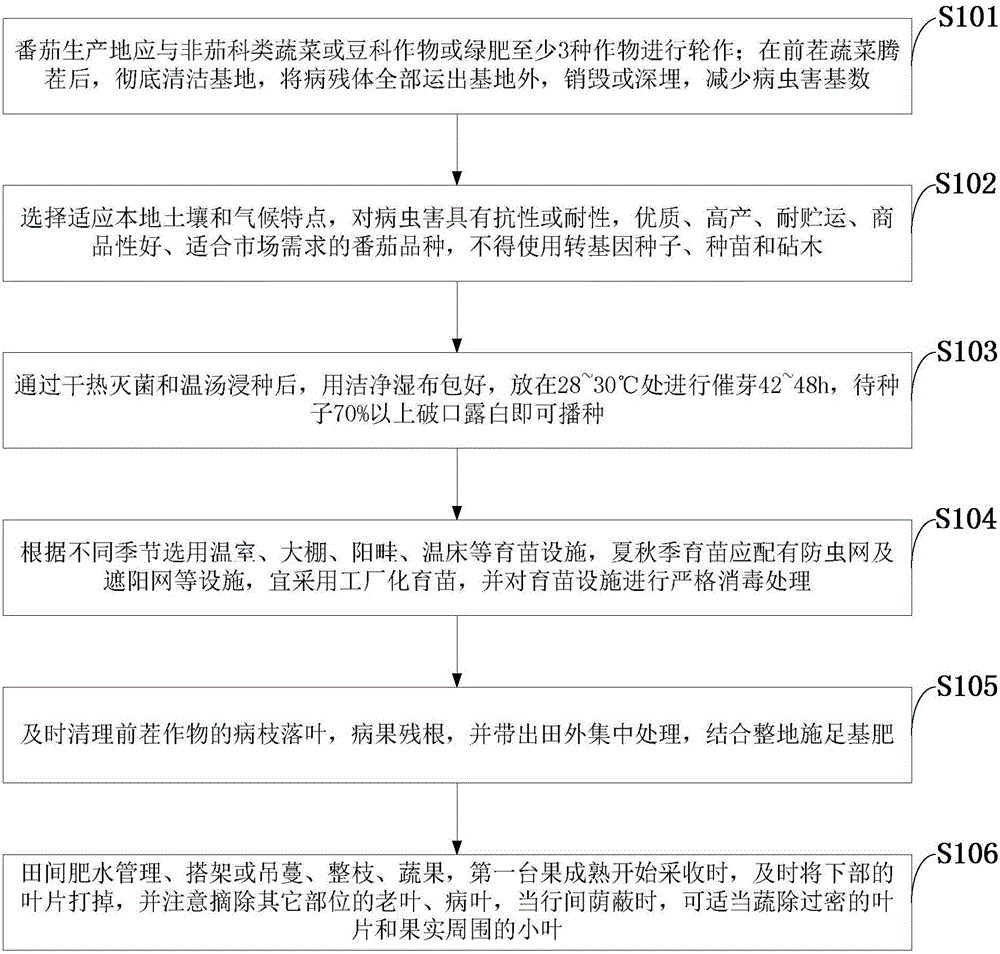
The invention discloses a method for a high yield of organic tomatoes.The method comprises the steps of establishment of a rotation system; variety selection; seed treatment, wherein seeds are subjected to dry heat sterilization and hot water immersion, wrapped in clean wet cloth and placed at the temperature of 28-30 DEG C for accelerating germination for 42-48 h, and sowing can be carried out when 70% or above of the seeds open and white buds are exposed; seedling growing, wherein seedling growing facilities such as a greenhouse, a shed, a cold frame and a hotbed are selected according to different seasons, an insect net and a shading net should be arranged for growing seedlings in summer and autumn, industrial seedling growing is adopted, and the seedling growing facilities are strictly disinfected; field management, wherein field water and fertilizer management, frame set-up or vine lifting, pruning and fruit management are included.The method for the high yield of organic tomatoes is beneficial to development of the organic agricultural industry, agricultural sustainable development is realized, a set of peculiar organic tomato production technology is formed, and a remarkable effect is achieved.
Owner:CHENGDU FANSHANG AGRI CO LTD
Method for separating and purifying alpha 1-antitrypsin from human blood plasma component FIV precipitation
ActiveCN101274956AHigh purityHigh yieldPeptide preparation methodsProtease inhibitorsFiltrationBlood plasma
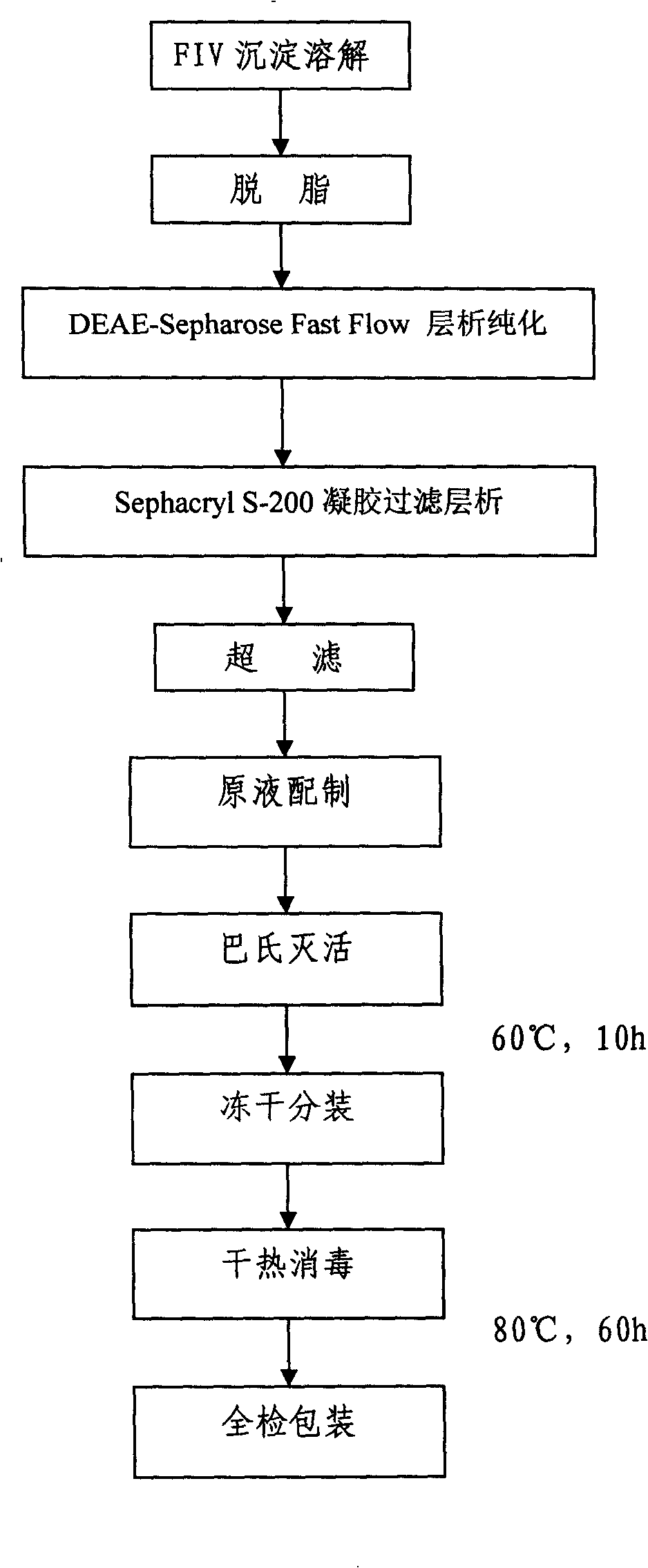
The invention discloses a method for separating and purifying alpha1-antitryptase from the FIV component precipitation of human plasma, which is characterized in that the method comprises the following steps of (A) pretreatment of plasma precipitation; (B) anion gel chromatography; (C) gel-filtration chromatography; (D) ultrafiltration desalting concentration; (E) Baculovirus inactivation; (F) lyophilization and subpackaging; (G) dry heat sterilization. The method takes the FIV component precipitation which is waste material produced from human plasma during the production process of human serum albumin as raw material and adopts chromatography technique to separate and purify a protease inhibitor, namely, alpha 1- antitryptase (alpha 1-AT) and builds the production technique of alpha 1-AT concentrate. Products prepared by the method has good pureness, high yield and simple and easy operation as well as few occupied equipment, little labor intensity, low energy consumption and low production cost due to taking the waste material generated by albumin as raw material.
Owner:广东双林生物制药有限公司
Sterilizable assembly bracket for sterile operation experiment
ActiveCN103343087AImprove accuracyImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsAlcoholCulture vessel
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Simple and efficient cultivation method convenient to implement for sugarcane seed seedlings
InactiveCN104365406AFast absorptionSave energyBioloigcal waste fertilisersGrowth substratesAgricultural engineeringDry Heat Sterilization
The invention discloses a simple and efficient cultivation method convenient to implement for sugarcane seed seedlings. According to the cultivation method, the number of set of equipment required by steam autoclaving sterilization or dry heat sterilization on a culture medium is reduced, and energy and labor are saved; meanwhile, bagasse is fully used as a part of the medium, and therefore the seedlings can quickly absorb required effective nutritional ingredients; besides, the cultivation method is simple in operation process and convenient to implement, low in cultivation cost, high in seedling survival rate, capable of shortening the cultivation time and high in operability and can be applied and popularized in sugarcane seedling cultivation in any sugarcane area.
Owner:广州甘蔗糖业研究所湛江甘蔗研究中心
New fermentation type coffee beans and preparation method thereof
The invention relates to new fermentation type coffee beans and a preparation method thereof. The preparation method comprises the following steps of (1) preparing bacillus natto liquid seeds: inoculating activated bacillus natto in a soybean flour liquid nutrient medium and performing culturing so as to obtain the bacillus natto liquid seeds at a logarithmic phase; (2) pretreating coffee beans: performing dry heat sterilization on the coffee beans and performing natural cooling; (3) fermenting the cooled coffee beans: inoculating the bacillus natto liquid seeds prepared in the step (1) to the sterilized coffee beans, and performing fermentation; and (4) stir-frying the fermented coffee beans: stir-frying the fermented coffee beans until the coffee beans are deeply baked, so that the fermentation type coffee beans are prepared. The prepared fermentation type coffee beans are low in content of caffeine, are favorable for human health, contain varied probiotic components such as natto kinase and SOD enzymes, and have the efficacies of strengthening the intestine and the stomach, resisting thrombosis, keeping young and the like.
Owner:GUANGZHOU BAIWO BIOTECH CO LTD
Sterilization process design for a medical adhesive
ActiveUS20150037200A1Effective sterilizationReduce the risk of infectionLavatory sanitoryMedical waste disposalAdhesiveEngineering
Medical devices, including medical adhesives, need to be sterile before application to a patient. A dry heat sterilization process can sterilize medical adhesives for patient application. The dry heat sterilization process can be validated for particular equipment arrangements and medical adhesives being utilized.
Owner:TYCO HEALTHCARE GRP LP
High-use-ratio manufacturing technology of extracting highly-active agarose product from red algae
The invention discloses a high-use-ratio manufacturing technology of extracting a highly-active agarose product from red algae. The low-alkali pressurizing method is adopted to pretreat the algae, so that the agaropectin extraction rate is effectively increased, and the high-alkali consumption is reduced; a multi-stage vibrating screen secondary agaropectin extraction technology is developed to increase the agaropectin extraction rate, and the residue is modified and processed to form manure or a feed additive, so that the comprehensive use rate of the red algae is greatly increased; a filter system, a novel band drying system, a full-automatic strip drying system for strip agar, a high temperature dry heat sterilization system and other novel integrated large-scale manufacturing devices are developed, so that the problems of high energy-consumption and lag in production technique in the actual production process are solved accordingly.
Owner:FUJIAN GOLD SWALLOW OCEAN BIOTECH
Turfgrass nematode control method
InactiveCN103004401AGuaranteed normal growthReduce densificationOrganic fertilisersHorticultureNematodeLitter
The invention discloses a turfgrass nematode control method which comprises the following steps: (1) preparation of turfgrass cultivation matrix: 5-15 cm deep fertile soil is evenly mixed, and is divided into two parts, wherein one part is used as inoculation soil, and the other part is disinfection soil which is implemented dry heat sterilization for two hours at the temperature of 160 DEG C; inactivated garbage composts and waste rubber particles with 0.5-1 mm particle size are additionally taken; the following raw materials are taken and are mixed evenly in parts by weight: 90-160 parts of inoculation soil, 140-260 parts of disinfection soil, 90-460 parts of garbage composts and 10-20 parts of waste rubber particles; (2) sowing of turfgrass; and (3) management and maintenance of turfgrass. The method can reduce soil compactness, bring convenience for movement of water in the soil, and modify water retaining property of the soil; and the garbage composts have an obvious restraining effect on plant parasitic groups, and can effectively kill and restrain breeding and growth of nematodes.
Owner:DASHUN INT FLOWER
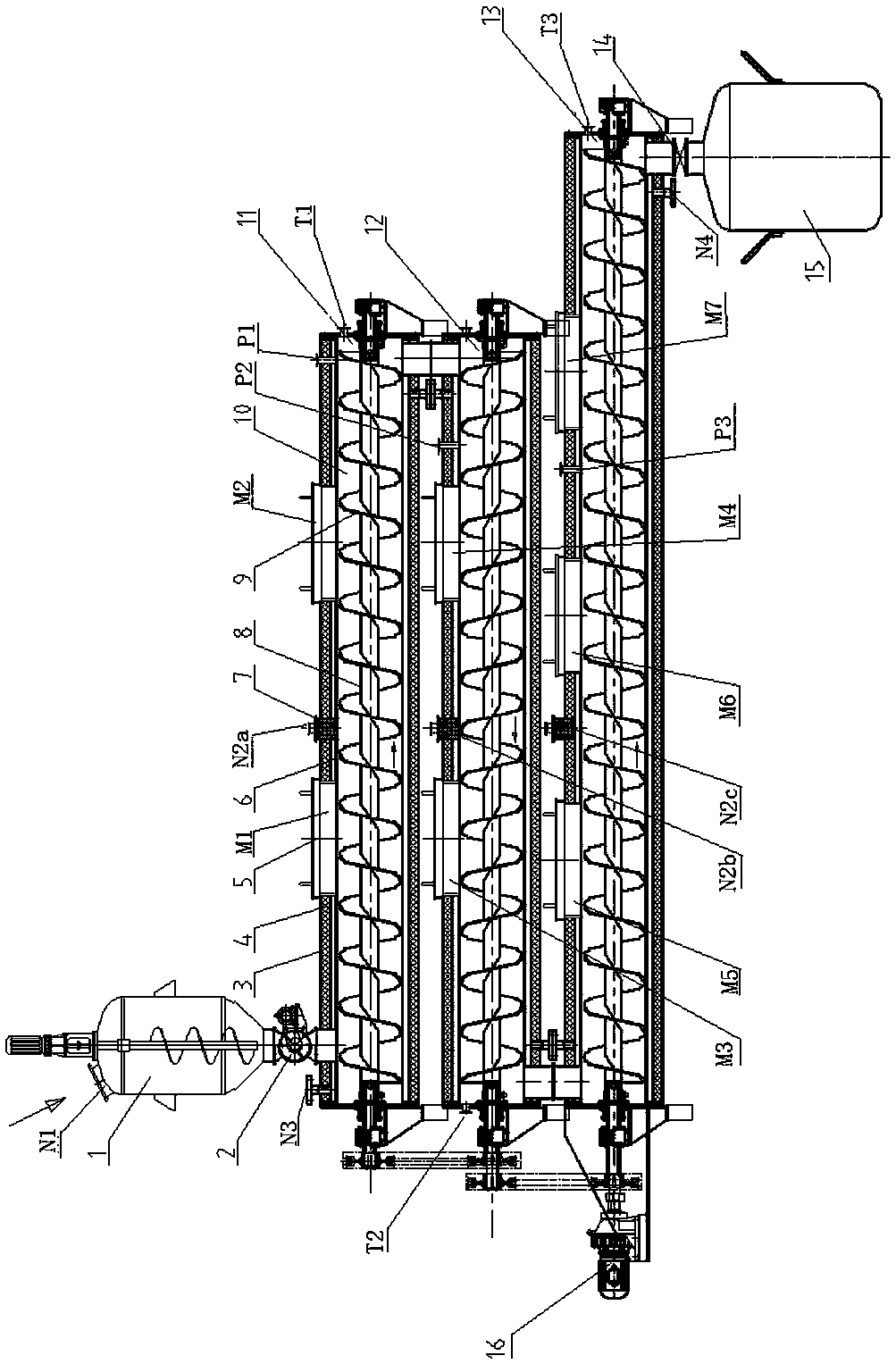
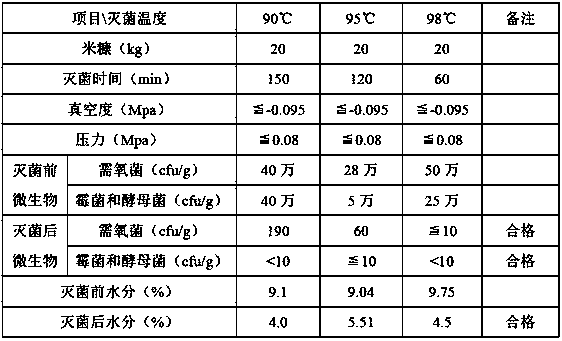
Dry-heat low-pressure continuous sterilization method
ActiveCN110522929AReduce the sterilization temperatureBreak through technical difficulties in the field of sterilizationLavatory sanitoryHeatPositive pressureEngineering
The invention belongs to the technical field of material sterilization. The invention relates to a dry-heat low-pressure continuous sterilization method. A sterilization inner cavity is heated througha heating device; the sterilization inner cavity is vacuumized through a vacuum device; the temperature of the sterilization inner cavity is adjusted and set during sterilization; materials are fed from one end of the tubular sterilization inner cavity, the material is conveyed from a feeding port to a discharging port through a conveying packing auger. In the sterilization process, the maintaining time of the vacuum degree and the pressure is controlled according to the number of microorganisms and the water content of materials, in the material conveying process, sterilization is conductedat the temperature lower than 100 DEG C under the positive-pressure and negative-pressure combined action, and the sterilization time is adjusted by adjusting the staying time of the materials in thesterilization inner cavity by adjusting the rotating speed of the conveying auger. A traditional dry heat sterilization mode is combined with preset positive and negative pressure, a physical sterilization process without any sterilizing agent works under a relatively low temperature condition, the medicine property of the materials is kept, and continuous sterilization is realized.
Owner:江西赫柏康华制药设备有限公司 +1
Steam sterilization system for sterilizing medical waste
Owner:LEWIS ROBERT W
Semi-continuous energy-efficient dry heat sterilization cabinet having sterile butt joint with RABS (Restricted Access Barrier System)
The invention discloses a semi-continuous energy-efficient dry heat sterilization cabinet having a sterile butt joint with an RABS. The cabinet is characterized in that: the cabinet comprises a sealed cabinet body and a vehicle track; one end of the sealed cabinet is provided with an vehicle entry door, and the other end of the sealed cabinet is provided with a vehicle exit door; an interval isolation door is transversely arranged in the sealed cabinet, a heat sterilizing chamber is arranged between the vehicle entry door and the interval isolation door, and a cooling chamber is arranged between the interval isolation door and the vehicle exit door; and the vehicle track which is arranged in the sealed cabinet body traverses the bottom of the interval isolation door, one end of the vehicle track reaches toward the bottom of the vehicle entry door, and the other end of the vehicle track reaches toward the bottom of the vehicle exit door. According to the present invention, the inner ofthe sealed cabinet body is divided into the heat sterilizing chamber and the cooling chamber, and the vehicle exit door of one side of the cooling chamber has a direct butt joint with the RABS, so the sterile butt joint between the sterilization cabinet and the RABS which is a usage point is satisfactorily solved, and risks brought by a case that inner packaging materials and appliances are artificially transferred to the RABS after being sterilized in sterile medicament production processes are avoided, thereby the cabinet allows sterile level and visible foreign matter level of products to be effectively controlled, energy to be saved, and efficiency to be high.
Owner:CHONGQING LUMMY PHARMA
Reusable nanofiber mask, protective film, and preparation method and application thereof
InactiveCN111227351AImprove the protective effectExtended service lifeProtective garmentSpecial outerwear garmentsPolyethylene terephthalate glycolPolyethylene glycol
The invention provides a reusable nanofiber mask, a protective film and a preparation method and application thereof. The mask protective film is composed of an isolation layer and a supporting layer.The isolation layer is composed of nano polytetrafluoroethylene fibers or nano cellulose fibers modified by a silane coupling agent, the quantification is 5-8 gsm, the thickness is 5-10 microns, andthe pore diameter is 100-200 nanometers. The supporting layer is composed of polyethylene glycol terephthalate fibers and double-melting-point PET fibers, the quantification is 20-30 gsm, the thickness is 50-80 micrometers, and the pore diameter is 3-20 micrometers. The mask protective film is formed by an isolation layer and a supporting layer through papermaking of a multi-flow-channel inclinedwire paper machine, or is obtained by hot-pressing compounding of the isolation layer and the supporting layer. The mask protective film is used in cooperation with a general mask, the isolation layeris attached to the face, the filtering efficiency of the mask exceeds 95%, and droplets are isolated. The mask protective film can be repeatedly used for 5-10 times after being subjected to ultraviolet or dry heat sterilization.
Owner:SOUTH CHINA UNIV OF TECH
Dry heat convection sterilization system
A dry heat sterilization system having a housing and a number of plenums to guide the flow of hot air. Hot air is forced to flow through the plenums, the intake slide duct and into the bottom of the containers. The hot air will rise and exit from the top of the container through the exhaust slide duct. Furthermore, the external surfaces of the container are also sterilized through the use of semi-pierced duct walls with adjustable diffuser panels. Hot air will re-circulate until it reaches a pre-determined temperature. The container and its contents can be safely handled once the exhaust air blower removes hot air from the system.
Owner:TPS
Method for preparing compound amino acid and ginseng saponin capsule
ActiveCN103989692ARelieve exercise fatigueImprove immunityOrganic active ingredientsAntinoxious agentsThreonineTyrosine
The invention discloses a method for preparing a compound amino acid and ginseng saponin capsule. The method comprises the following steps: (1), wherein the capsule is prepared from the following raw materials in parts by weight: 1-2 parts of compound ginseng saponin and 416-832 parts of compound amino acid, wherein the compound amino acid is prepared from the following components by mass percent: 1.22% of histidine, 1.02% of isoleucine, 1.43% of leucine, 1.22% of lysine, 1.32% of methionine, 0.51% of tyrosine, 0.81% of phenylalanine, 0.36% of threonine, 1.02% of valine, 14.58% of alanine, 11.93% of arginine, 13.16% of aspartic acid, 5.3% of glycine, 26.11% of glutamic acid, 6.63% of proline and 3.66% of serine; the compound ginseng saponin is prepared from the following components by mass percent: 14.8% of Rgl, 11.73% of Re, 28.21% of Rbl, 1.40% of Rg2, 20.11% of Rc, 16.48% of Rb2 and 7.26% of Rd; (2) fully mixing the compound amino acid with the compound ginseng saponin evenly according to the raw materials in the step (2), carrying out dry heat sterilization at 100 DEG C for an hour, and enclosing into capsules after sterilizing, so as to prepare the product.
Owner:中农合裕(辽宁)农业科技有限公司
Dry heat sterilization indicator and preparation method thereof
ActiveCN106362180AMeet the needs of dry heat sterilizationMeets standards for sterilization applicationsLavatory sanitoryInvestigating sterilization degreeMANNITOL/SORBITOLMixed materials
The invention discloses a dry heat sterilization indicator and a preparation method of the dry heat sterilization indicator, belonging to the field of dry heat sterilization indication. The dry heat sterilization indicator is prepared from the following active ingredients in parts by weight: 1-10 parts of methylamino anthraquinone, 1-10 parts of polyvinylpyrrolidone, and 50-90 parts of mannitol. The preparation method of the dry heat sterilization indicator comprises the following steps: placing the active ingredients into a mixer, uniformly mixing the active ingredients so that a mixed material is formed, feeding the mixed material into a tablet press, and carrying out tabletting, thus obtaining sterilization indication tablets; placing the indication tablets into a device with a water-absorbing paper core, and sealing the device, thus obtaining the dry heat sterilization indicator. For the dry heat sterilization indicator provided by the invention, when exposed under the 160-180 DEG C dry heat condition, the indication tablets melt and creep along the water-absorbing paper core, and then whether the sterilization is qualified or not can be judged according to the creeping distance.
Owner:NANJING JUSHA DISPLAY TECH
Method for preserving microbial strains for long time
InactiveCN102399696AReduce the number of passagesReduce the chance of mutationMicroorganism based processesMicroorganism preservationAcetic acidMicroorganism
The invention relates to a microbial strain preserving method, in particular to a method for preserving microbial strains for a long time. The method comprises the main steps of: inoculating the strains onto a slant culture medium; cultivating the strains for 20-30 hours at a temperature of 20-40 DEG C; adding a little calcium carbonate onto a slope, wherein the calcium carbonate is subjected to dry heat sterilization; filling a liquid tubular culture medium into a test tube; plugging the test tube by using a sterile rubber plug; and putting the test tube in a refrigerator for preservation at a temperature of 2-4 DEG C. When the method is adopted to preserve acetic acid strains, reproducing times of the acetic acid strains can be reduced, namely mutation probability can be reduced. The method is simple to operation; the preservation period can reach 2 years; and the microbial strains can be put into production after being activated by using the slant culture medium for two times when used, without complex operation processes. In addition, the purpose of preservation can be achieved without any expensive instrument; the method is suitable for mass production; and according to the method, cost can be lowered, and energy consumption can be reduced.
Owner:SHANGHAI HAIDI GARDENING
Method for measuring inhibition zone of yellow rice wine by filter paper dispersion method
The invention relates to a method for measuring the inhibition zone of yellow rice wine by a filter paper dispersion method, which comprises the following steps of: (1) bacterial solution preparation: inoculating escherichia coli and staphylococcus aureus strains on the inclined plane of a beef extract-peptone culture medium, picking bacteria from the inclined plane to 100 mL of sterile water by an inoculating loop to prepare the bacterial solution, and plugging by tampon for later use; (2) preparation and treatment of filter paper: punching round filter paper by a puncher, performing dry heat sterilization for later use, clamping the sterilized filter paper by forceps and soaking the filter paper into medicine liquid and wine samples with different concentrations for later use; and (3) inoculation and cultivation: preparing a flat plate by the sterilized beef extract-peptone culture medium under the aseptic condition, adding bacteria for testing after cooling and solidification, coating uniformly by an aseptic coating device, clamping the filter paper, which is subjected to extraction treatment, by aseptic forceps, and adhering the filter paper to a bacterium-containing plate. By the method, the inhibition effect of mildew and bacteria can be controlled, so that a theoretical basis and a technical support are provided for production, research and development of the functional yellow rice wine.
Owner:SHAOXING UNIVERSITY
Seed uniform dry heat sterilization treatment equipment and control method
PendingCN108738497AMeet dry heat treatmentEasy to controlSeed immunisationTemperature controlProcess engineering
The invention relates to seed uniform dry heat sterilization treatment equipment and a control method. The seed uniform dry heat sterilization treatment equipment is formed by a shell, a seed circulating transportation system, a temperature and humidity regulating system, a seed uniform dry heat system, an intelligent controller, a temperature sensor, a humidity sensor and related components. Theseed uniform dry heat sterilization treatment equipment disclosed by the invention is capable of controlling a heating temperature to change according to a preset temperature-time curve, meanwhile, uniformity of the temperature of environment of seeds can be realized, and accurate control on temperature in different positions can be realized during a temperature change process. The seed uniform dry heat sterilization treatment equipment disclosed by the invention is consistent in seed treatment temperature, complete in sterilization, accurate in temperature control, high in efficiency and simple in operation, can meet dry heat treatment of various seeds and has wide adaptability.
Owner:NANJING AGRI MECHANIZATION INST MIN OF AGRI
Pigeon egg white powder and preparation method thereof
PendingCN112914048ASimple preparation processMild conditions for enzymatic hydrolysisEggs preservationFood dryingBiotechnologyAnimal science
The invention relates to the technical field of pigeon egg deep processing methods, in particular to pigeon egg white powder and a preparation method thereof. The pigeon egg white powder is obtained by the following steps: adding a compound enzyme into pigeon egg white for enzymolysis to obtain an enzymolysis product, then sequentially performing filtering, performing pasteurizing, performing spray-drying, performing dry heat sterilization, performing cooling and performing sieving on the enzymolysis product to obtain the pigeon egg white powder. The preparation method is simple in preparation process, mild in enzymolysis condition, and high in drying speed, the pigeon egg white powder is uniform and consistent in white color and luster, powdery fine particles are good in restorability, easy to absorb, short in dissolution time, good in dispersity and free of agglomeration phenomenon, and the problems that existing fresh eggs are prone to deterioration and breakage and are not beneficial to storage can be effectively solved.
Owner:新疆维吾尔自治区药学会 +1
Fermentation feed for aquatic products, preparation method thereof and application thereof
InactiveCN108065113ASimple processEasy to get materialsFood processingClimate change adaptationBiotechnologyDigestion
The present invention discloses a preparation method of a fermentation feed for aquatic products. The preparation method comprises the following steps: 1) bran, soybean meal and corn gluten meal in ratios are weighed, the weighed materials are subjected to dry heat sterilization, and the sterilized materials are crushed to prepare a raw material carrier; 2) mineral substances, koji seeds, carbon sources and plant vitamins are respectively taken, the taken materials are crushed, and the crushed materials are mixed to prepare a strain nutrient agent; 3) the raw material carrier is added into thestrain nutrient agent to be mixed and stirred to be prepared into a mixed feed; 4) water is added into the mixed feed for a sealed fermentation for 1-5 days to be prepared into a wet fermented material; and 5) according to different culture stages of shrimps, the wet fermented material is mixed with a full-price compound feed to be prepared into the fermentation feed for the aquatic products. Thepresent invention also discloses the fermentation feed for the aquatic products and an application thereof. The feed is solid, and convenient in circulation and transportation, greatly improves nutritional levels and digestion and utilization of the fermented material, and is safe.
Owner:佛山市珠水生物科技有限公司 +1
Method for preparing blood-activating tendon-strengthening pills for stabilizing content of dracorhodin in dragon blood
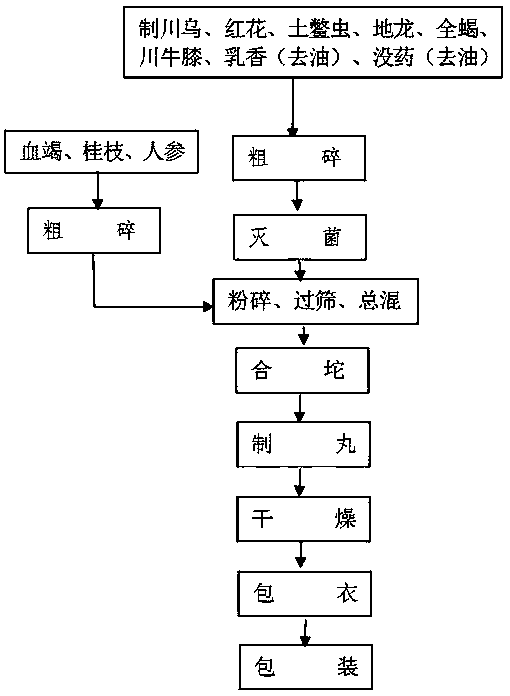
InactiveCN108938866AStabilized hematogenin contentGuaranteed efficacyAnthropod material medical ingredientsAntipyreticMyrrhGround beetle
The invention relates to a method for preparing blood-activating tendon-strengthening pills for stabilizing the content of dracorhodin in dragon blood. The method comprises the following steps: S1, coarsely crushing, namely coarsely crushing radix aconiti preparata, safflower carthamus, ground beetles, earthworms, scorpions, radix cyathulae, frankincense and myrrh in a coarse crusher to prepare mixed particles A, coarsely crushing dragon blood, cassia twigs and ginseng in a crusher to prepare mixed particles B; S2, sterilizing, namely sterilizing the mixed particles A in a dry heat sterilization cabinet; S3, crushing, screening and totally mixing, namely respectively putting the mixed particles B and the mixed particles A in the crusher to prepare medicinal powder B and medicinal powder A,screening, and mixing in a mixer to prepare a mixed powder preparation; S4, massing and pelletizing, namely mixing the powder preparation with pure water for 10 minutes, pressing in a pelletizing machine to prepare pill semi-finished products; S5, drying, namely drying the pill semi-finished products in a traditional Chinese medicine drying machine; S6, coating, coating the pill semi-finished products in a coating machine to prepare pills; and S7, packaging, namely packaging the pills, and storing in a warehouse.
Owner:HENAN RUNHONG HERBAL PHARMA
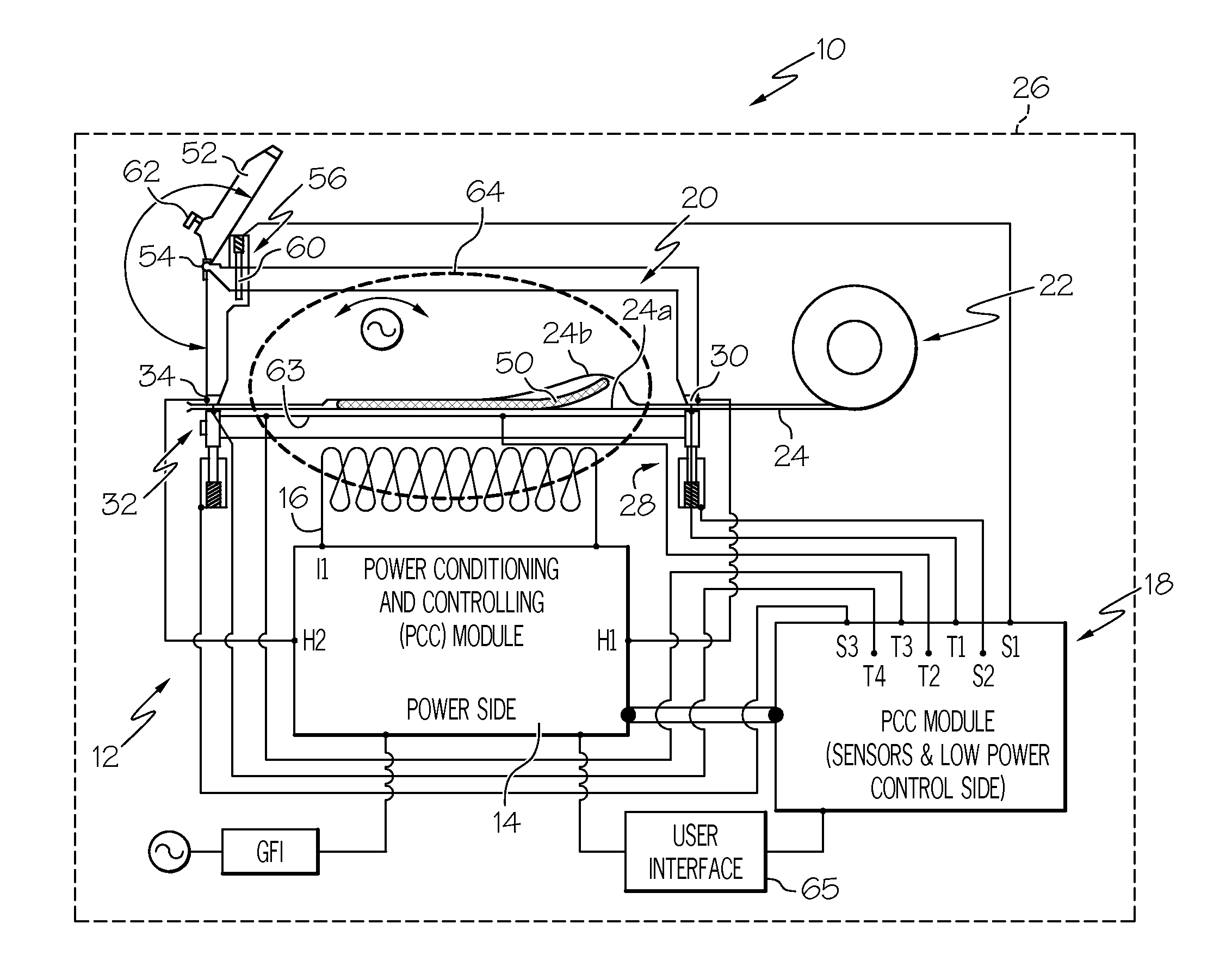
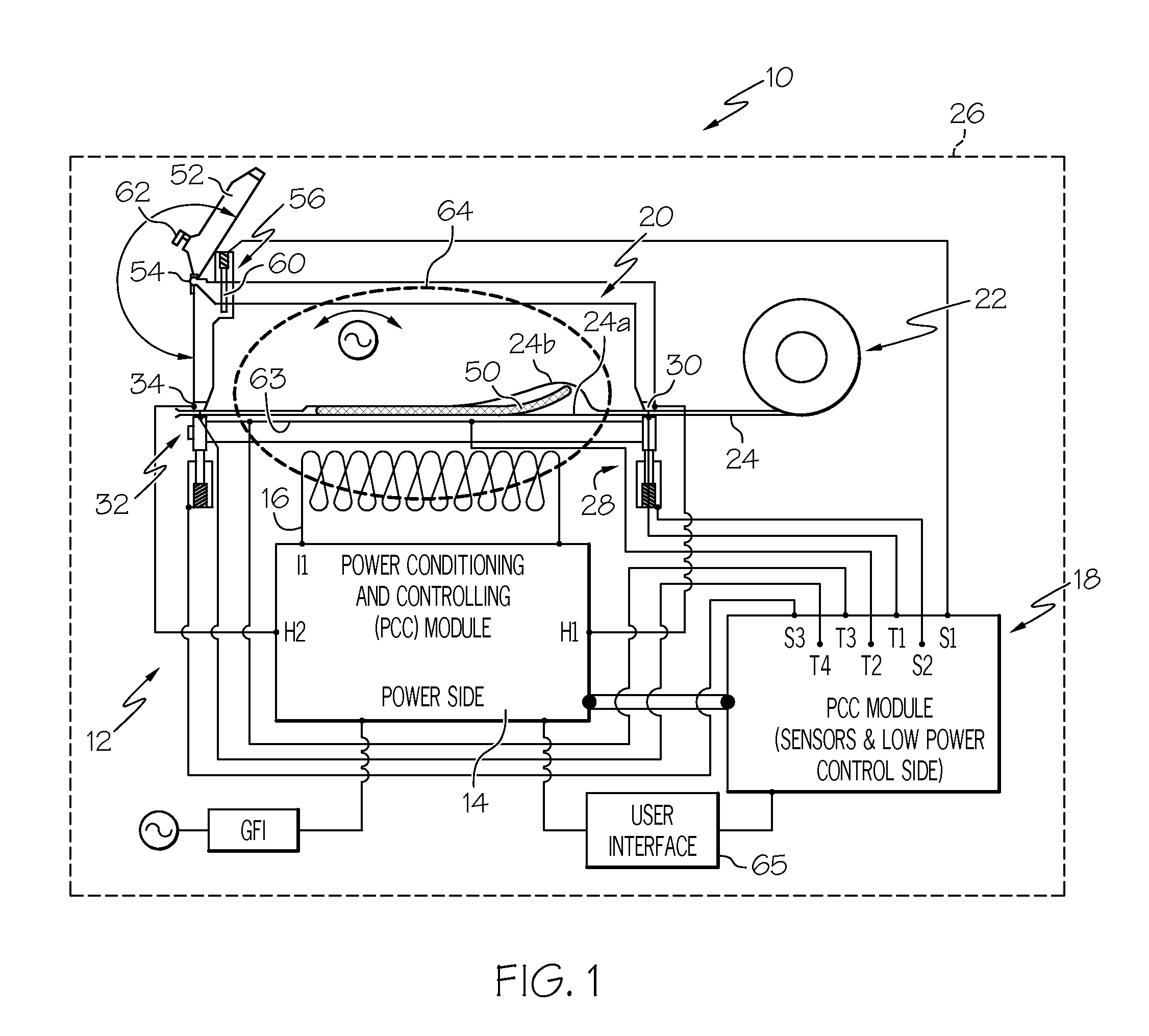
Dual-pass-through countertop high velocity hot air sterilizer
ActiveUS20190255202A1Reduce high temperatureEven heat distributionHeatAir quality improvementAir velocitySTERILE FIELD
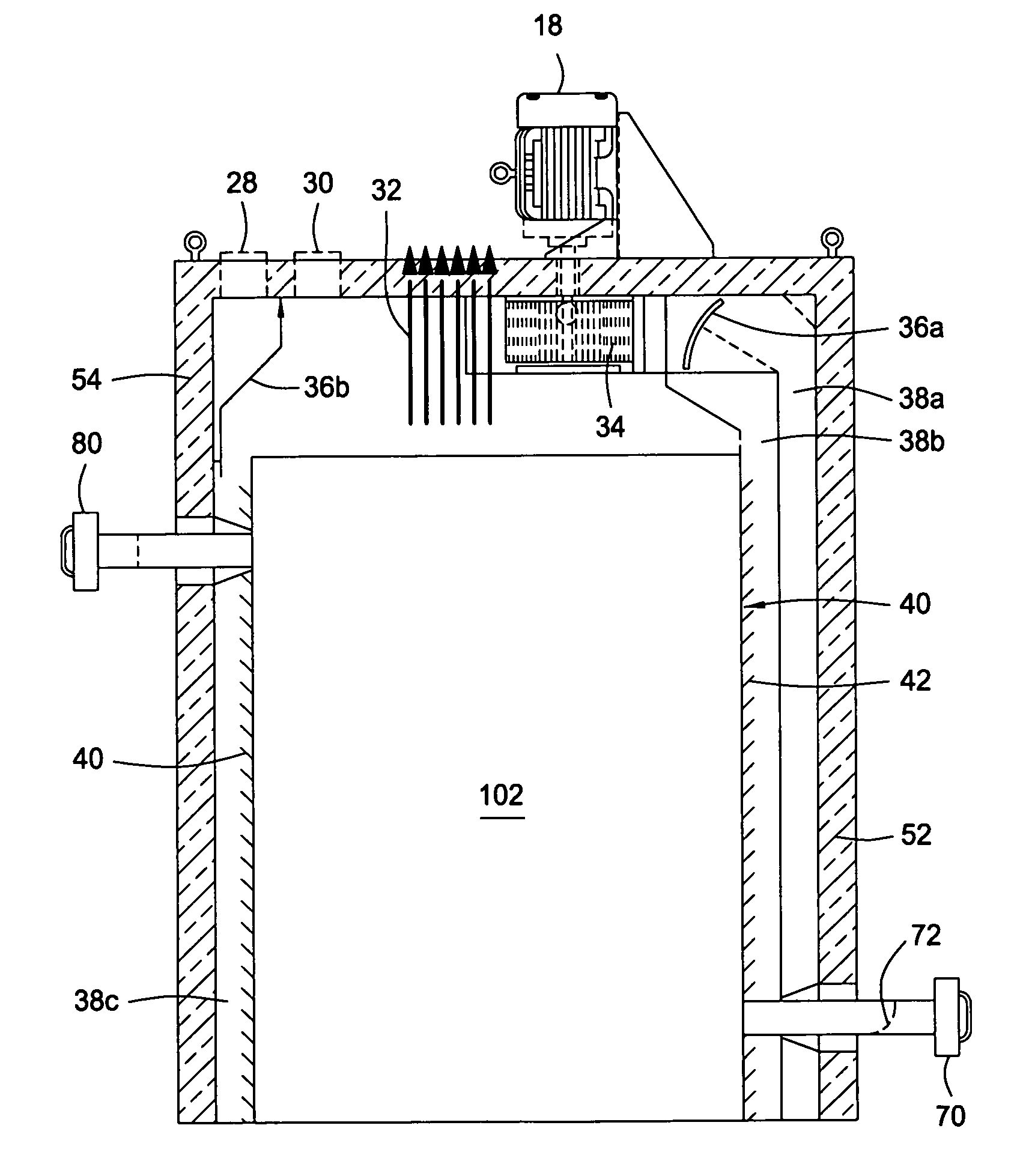
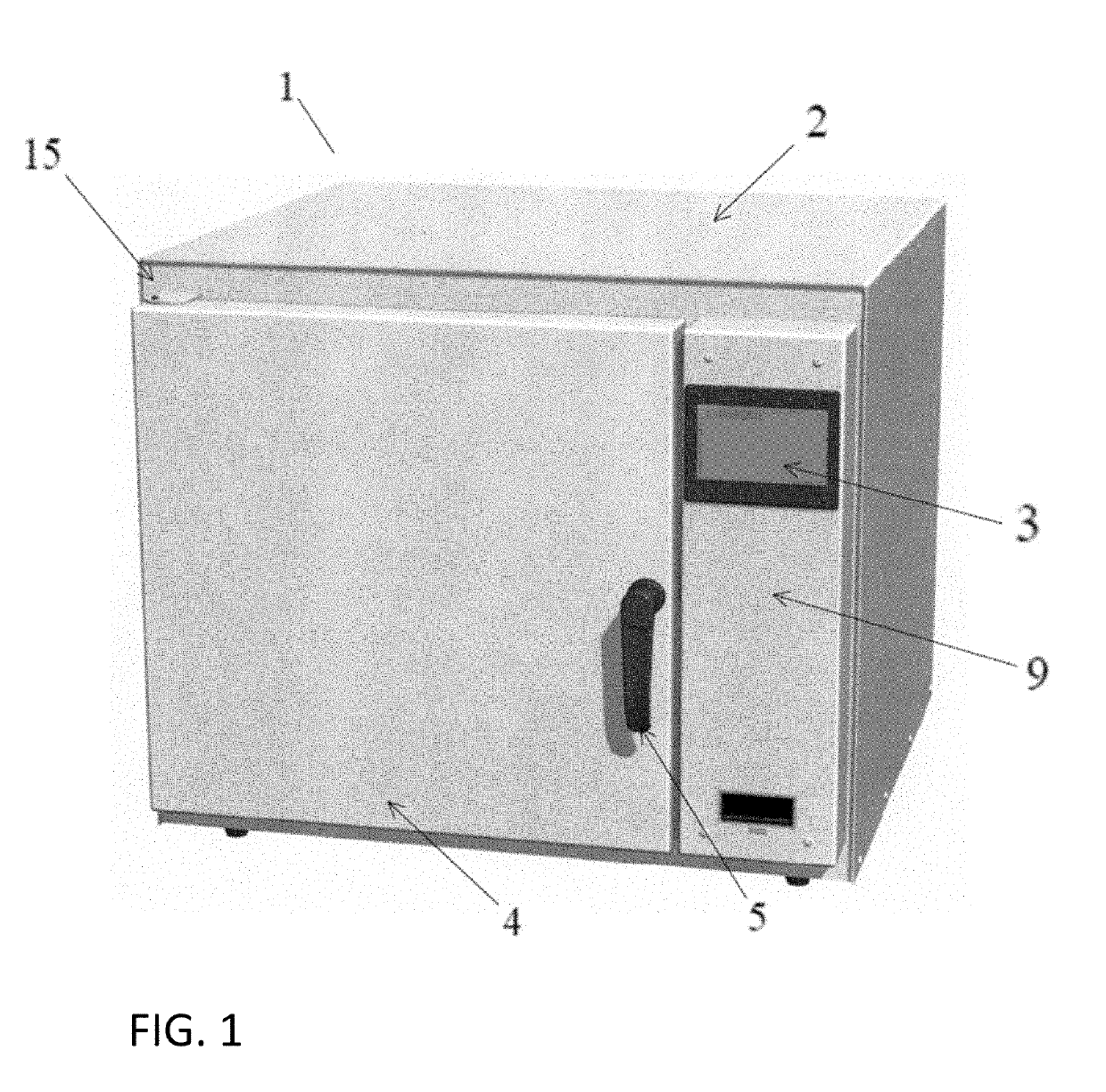
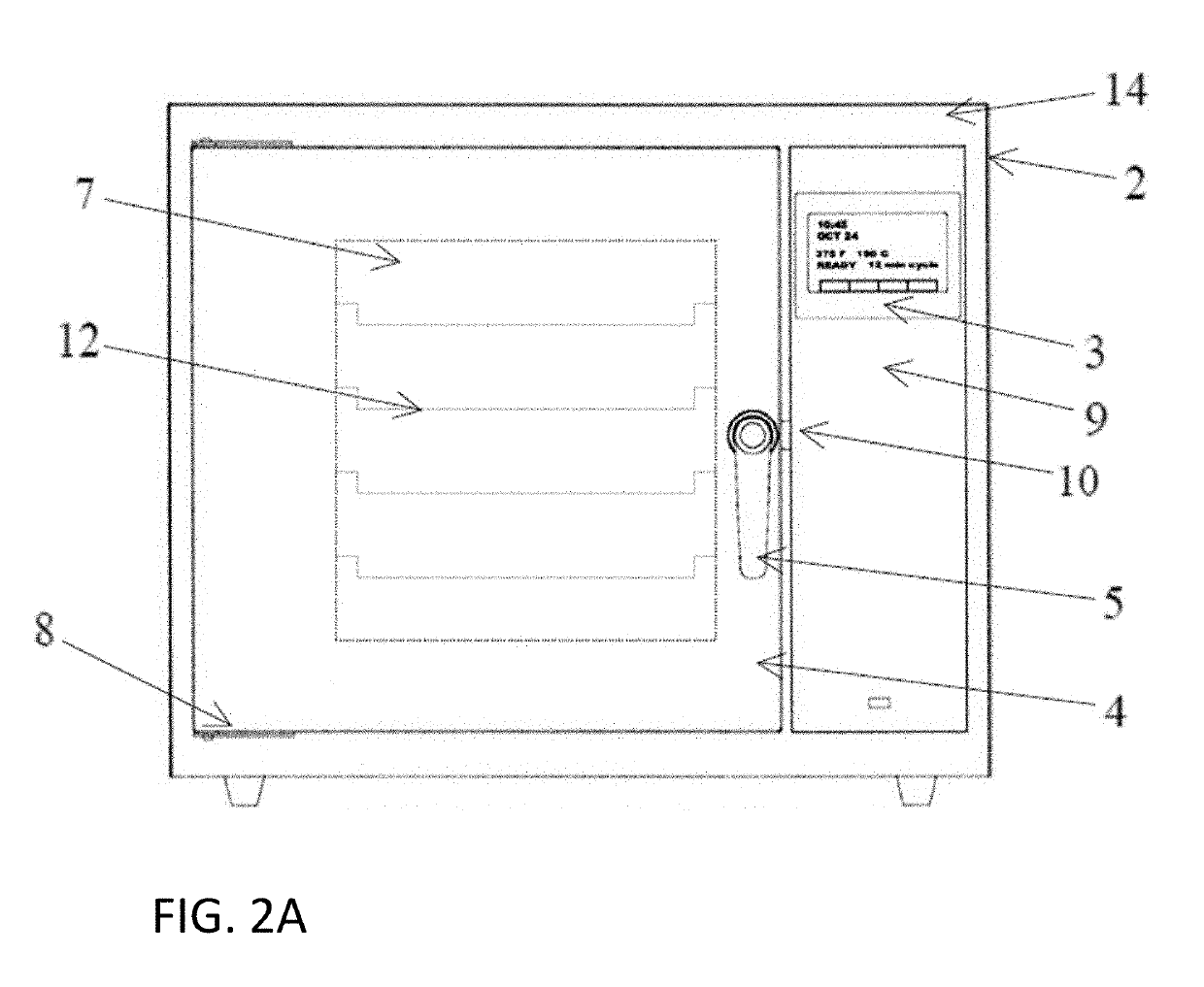
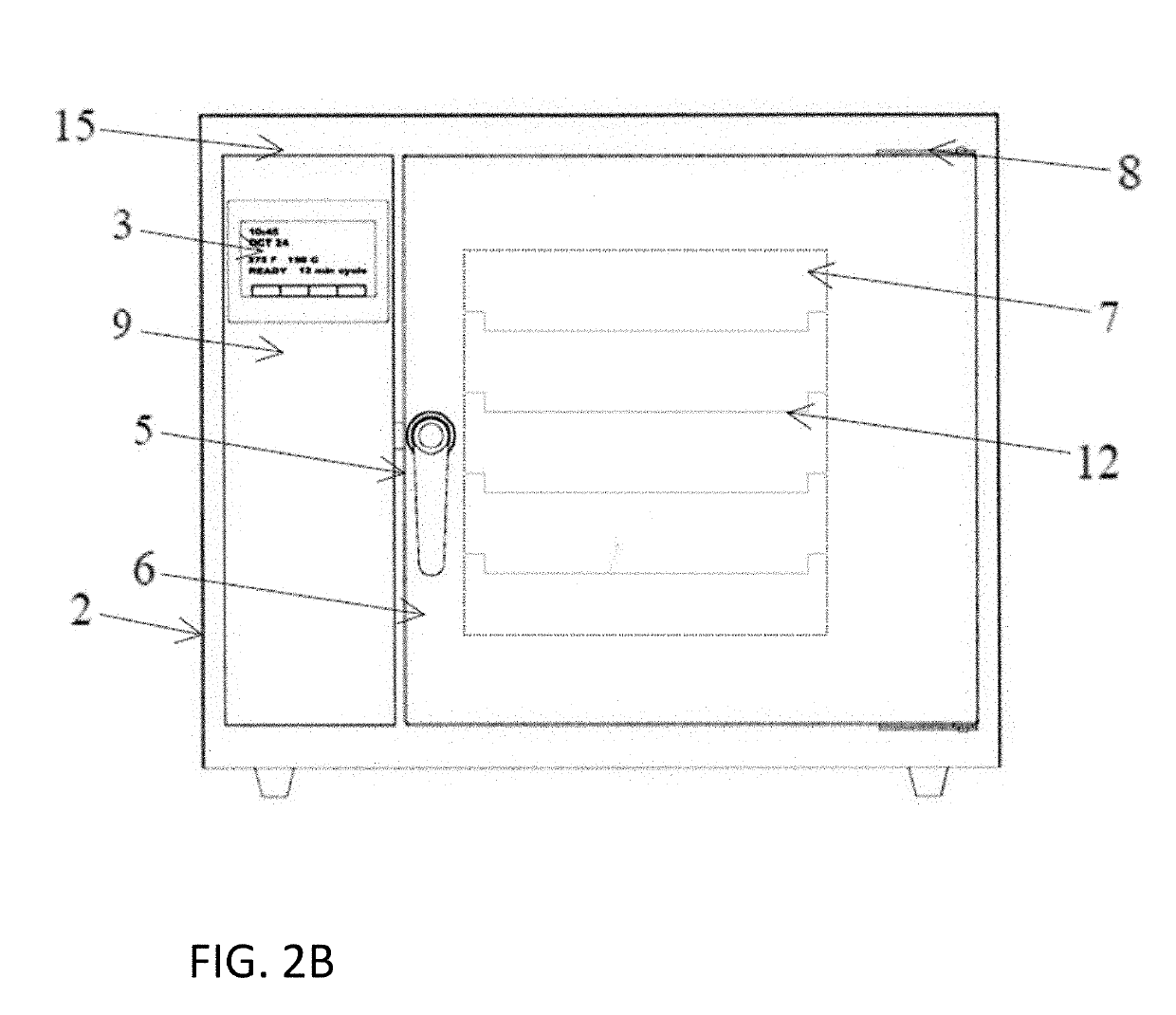
A device and system for sterilizing objects, commonly dental, medical, or veterinary instruments, having a double door configuration that can provide entry of contaminated instruments into a sterilizer from a contaminated area and subsequently, post-sterilization, their pass-through directly into a sterile area through an exit door from said sterilizer. More specifically, the invention is a countertop, high velocity, dry heat sterilization device that is readily adaptable for those clinical areas requiring separation of contaminated and sterile areas for the processing of medical, dental, or veterinary instruments. The sterilization device utilizes a dual airflow pathway and a controlled heat delivery system to deliver to the sterilization chamber the supply air having temperature uniformity and air velocity required of the high velocity dry heat sterilization process.
Owner:INTEGRATED MEDICAL TECH
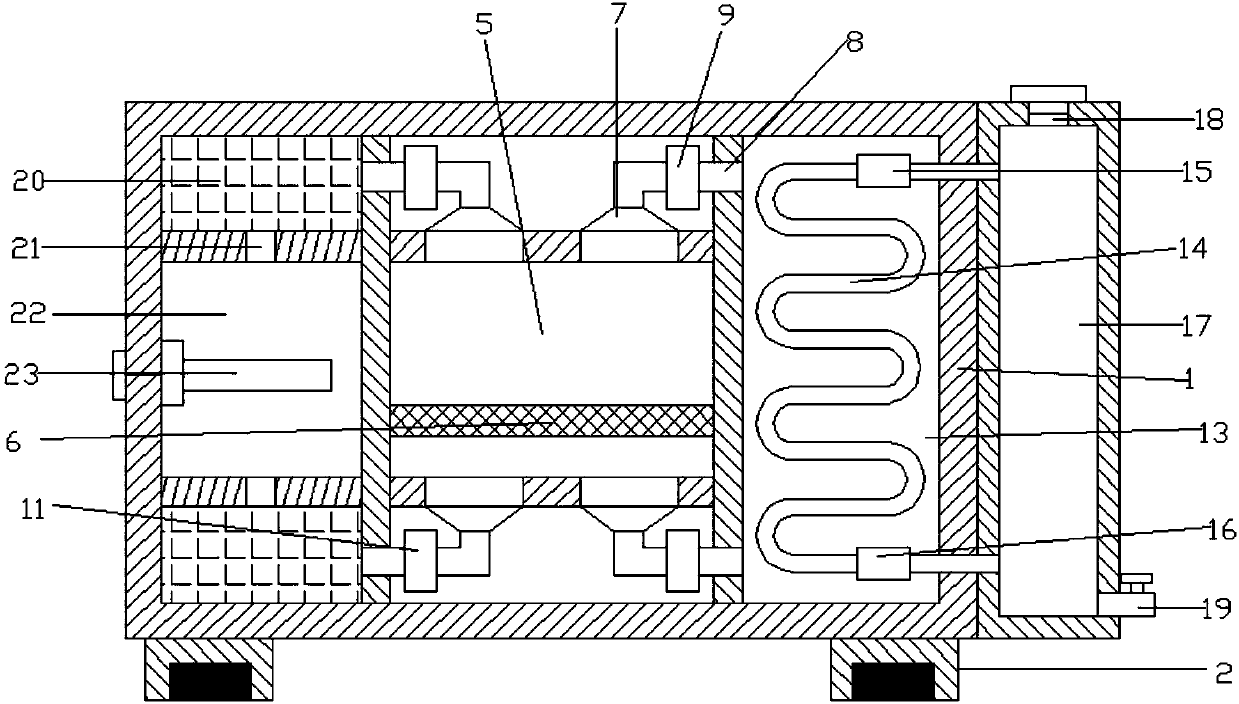
Medical dry-heat sterilization equipment
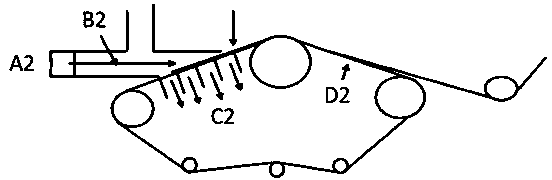
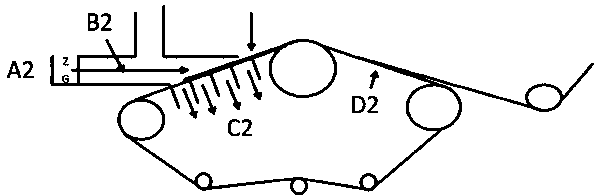
The invention discloses medical dry-heat sterilization equipment. The medical dry-heat sterilization equipment comprises a casing, a sterilization chamber is arranged in the casing, a supporting network plate is arranged at a lower end in the sterilization chamber, connection covers are arranged at a left end and a right end of an upper side and a lower side of the sterilization chamber, connecting pipes are arranged outside the connection cover, air-extracting pumps are respectively arranged at the connecting pipes on the upper end, air-discharging pumps are respectively arranged at the connecting pipes on the lower end, a condensation chamber is arranged at the right side of the connecting pipe on the right end, a spiral condenser tube is arranged in the condensation chamber, the air-extracting pump and the air-discharging pump are respectively arranged at an upper end and a lower end of the spiral condenser tube, a water-storage chamber is arranged at the right side of the condensation chamber, a water-discharging pipe is arranged at the lower end of the right side of the water-storage chamber, a drying chamber is arranged at the left side of the connecting pipe at the left end, air exhaust openings are arranged at the inner side of the drying chamber, a heating chamber is arranged between the air exhaust openings, and a heating rod is arranged in the heating chamber. The medical dry-heat sterilization equipment can effectively perform dry-heat cycle disinfection on the medical equipments, guarantees sterilization effect and sterilization efficiency of the equipment, and conveniently performs rapid cooling, so that equipment usage is convenient.
Owner:ZHENGZHOU RENHONG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com