Preparation method of triamcinolone acetonide
A compound and solvent technology, applied in the field of chemical synthesis of medicines, can solve the problems of high price of tetraene acetate and high cost of triamcinolone acetonide, simplify the operation of multi-step protection and deprotection, shorten the synthesis route, The effect of improving yield and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
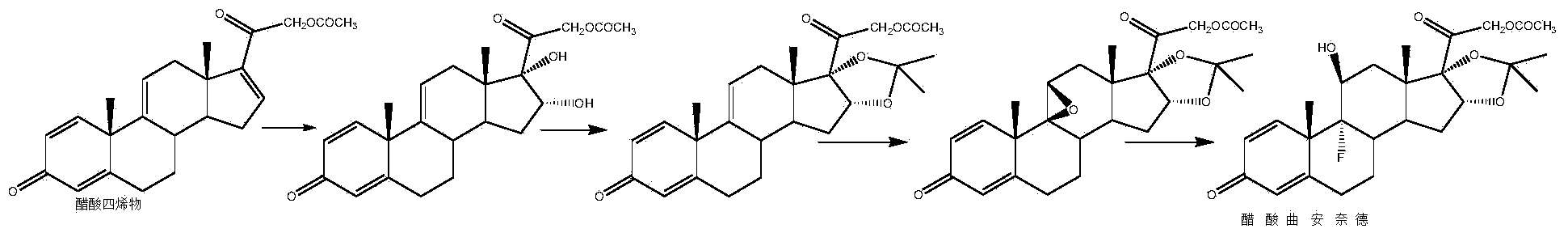
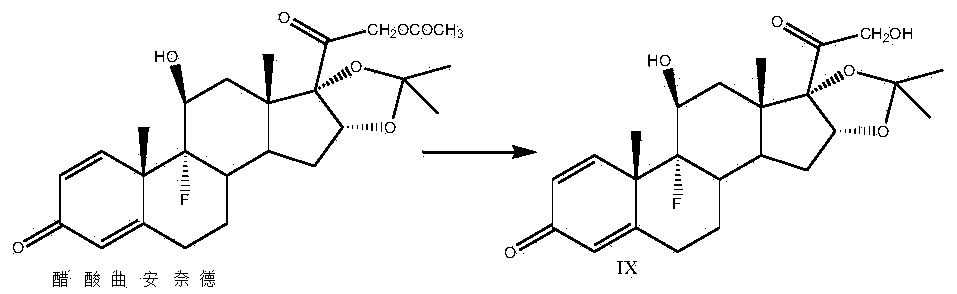
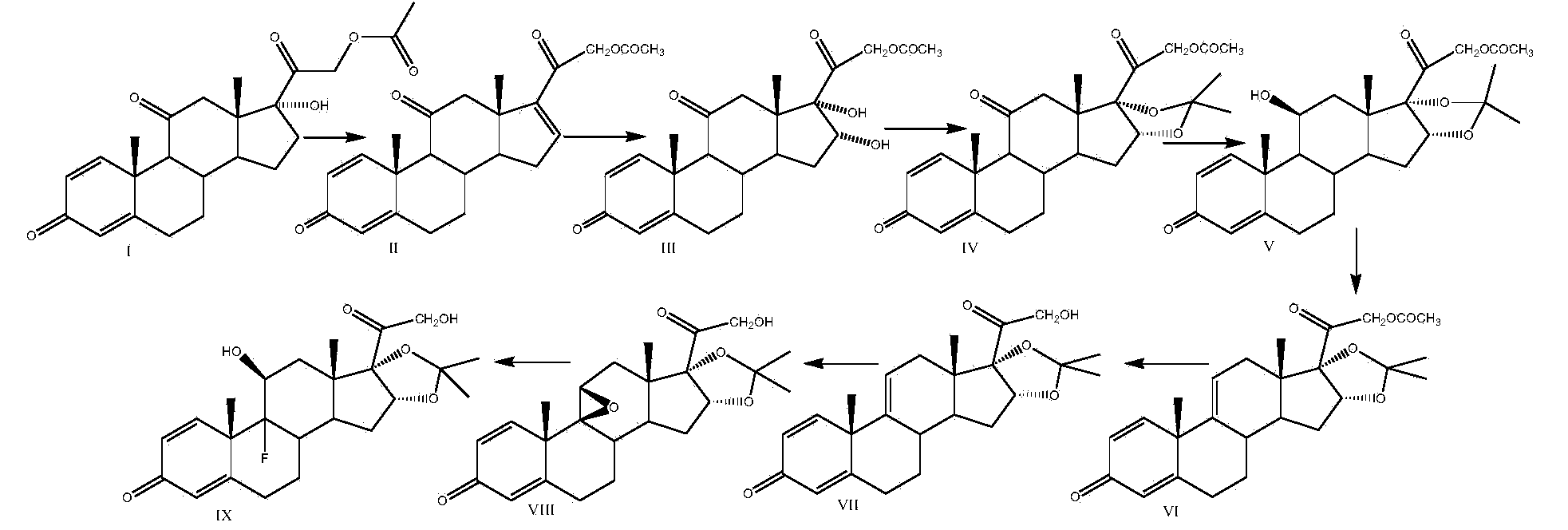
[0035]Take a three-necked reaction bottle, pass it into nitrogen protection, add 300ml of 2-picoline and 100g of prednisone acetate into the reaction bottle, stir at room temperature for 10-15min, cool down to -20°C, add pentachlorohydrin to the reaction solution in batches Phosphate 20g, stirred for 10-15min, then 20g of sulfur dioxide was introduced into the reaction bottle, the temperature was kept at -20-10°C, and the time was about 1.5-2h. Finally, 2500ml of water was slowly dropped into the reaction solution, filtered, washed with a small amount of water until neutral, and the solid was dried at 60°C to obtain 92g of compound II, namely 21-hydroxypregna-1,4,16(17)-tri En-3,11,20-trione 21-acetate, mass yield: 92%, HPLC purity: 98%.
[0036] Add 1000ml of acetone and 50g of compound II to the reaction bottle, dissolve under stirring at room temperature, add 10ml of formaldehyde, cool down to -10~-5°C, add potassium permanganate solution (weigh 24g of potassium permanganat...
Embodiment 2
[0044] Take a three-necked reaction flask, pass it through nitrogen protection, add 300ml of pyridine and 100g of prednisone acetate into the reaction flask, stir at room temperature for 10-15min, cool down to 15°C, add N-bromosuccinimide to the reaction solution 54g, stirred for 10-15min, put 20g of sulfur dioxide into the reaction flask, kept the temperature at 10-15°C, and took about 1.5-2h. 2500ml of water was slowly dropped into the solution, filtered, washed with a small amount of water until neutral, and the solid was dried at 60°C to obtain 95g of compound II, namely 21-hydroxypregna-1,4,16(17)-triene-3,11 , 20-triketone 21-acetate, mass yield: 95%, HPLC purity: 98%.
[0045] Add 1000ml of acetone and 50g of compound II to the reaction flask, dissolve under stirring at room temperature, add 15ml of acetic acid, cool down to 5-10°C, add potassium permanganate solution (weigh 24g of potassium permanganate into 600ml of water, heat up slightly to About 30°C, stir and dis...
Embodiment 3
[0053] Take a three-necked reaction bottle, pass it through nitrogen protection, add 400ml of diisopropylamine and 100g of prednisone acetate into the reaction bottle, stir at room temperature for 10-15min, cool down to -10°C, add N-chlorobutane di Imide 50g, stirred for 10-15min, passed 20g of sulfur dioxide into the reaction flask, kept the temperature below -10-5°C, and took about 1.5-2h. After completion, slowly drop 2500ml of water into the reaction solution, filter, wash with a small amount of water until neutral, and dry the solid at 60°C to obtain 94g of compound II, namely 21-hydroxypregna-1,4,16(17)- Triene-3,11,20-trione 21-acetate, mass yield: 94%, HPLC purity: 98%.
[0054] Add 1000ml of methyl ethyl ketone and 50g of compound II to the three-necked reaction flask, pass through nitrogen, dissolve under stirring at room temperature, add 8ml of formic acid, cool down to 10-15°C, add potassium chlorate solution (weigh 40g of potassium chlorate into 600ml of water, S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com