Ocular delivery of triamcinolone acetonide phosphate and related compounds
a technology of triamcinolone acetonide and related compounds, applied in the field of ocular delivery of triamcinolone acetonide phosphate and related compounds, can solve the problems of complex treatment of intermediate and posterior uveitis, systemic toxicities, and blindness of uveitis, and achieve the effect of treating or preventing an ocular condition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
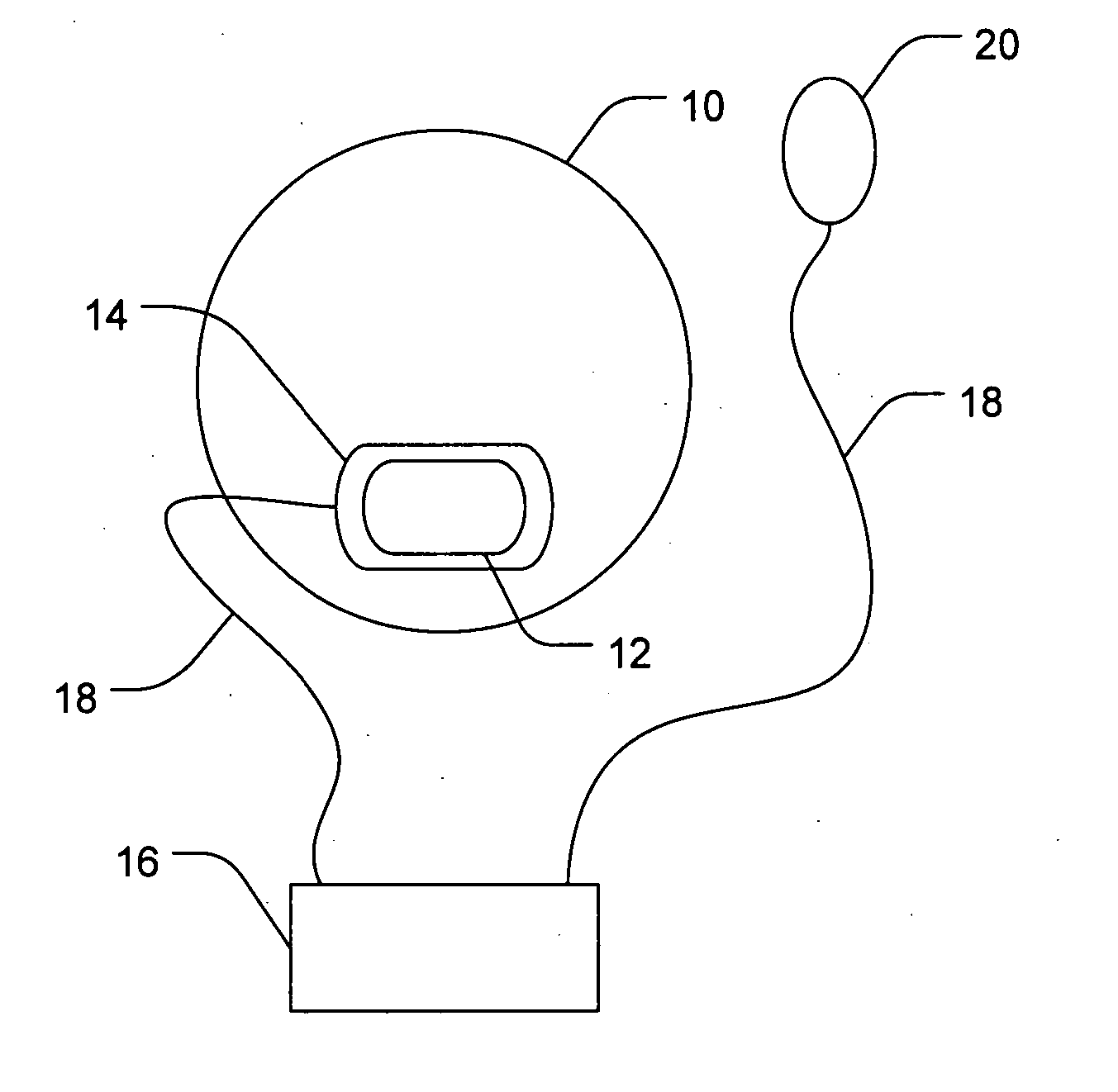
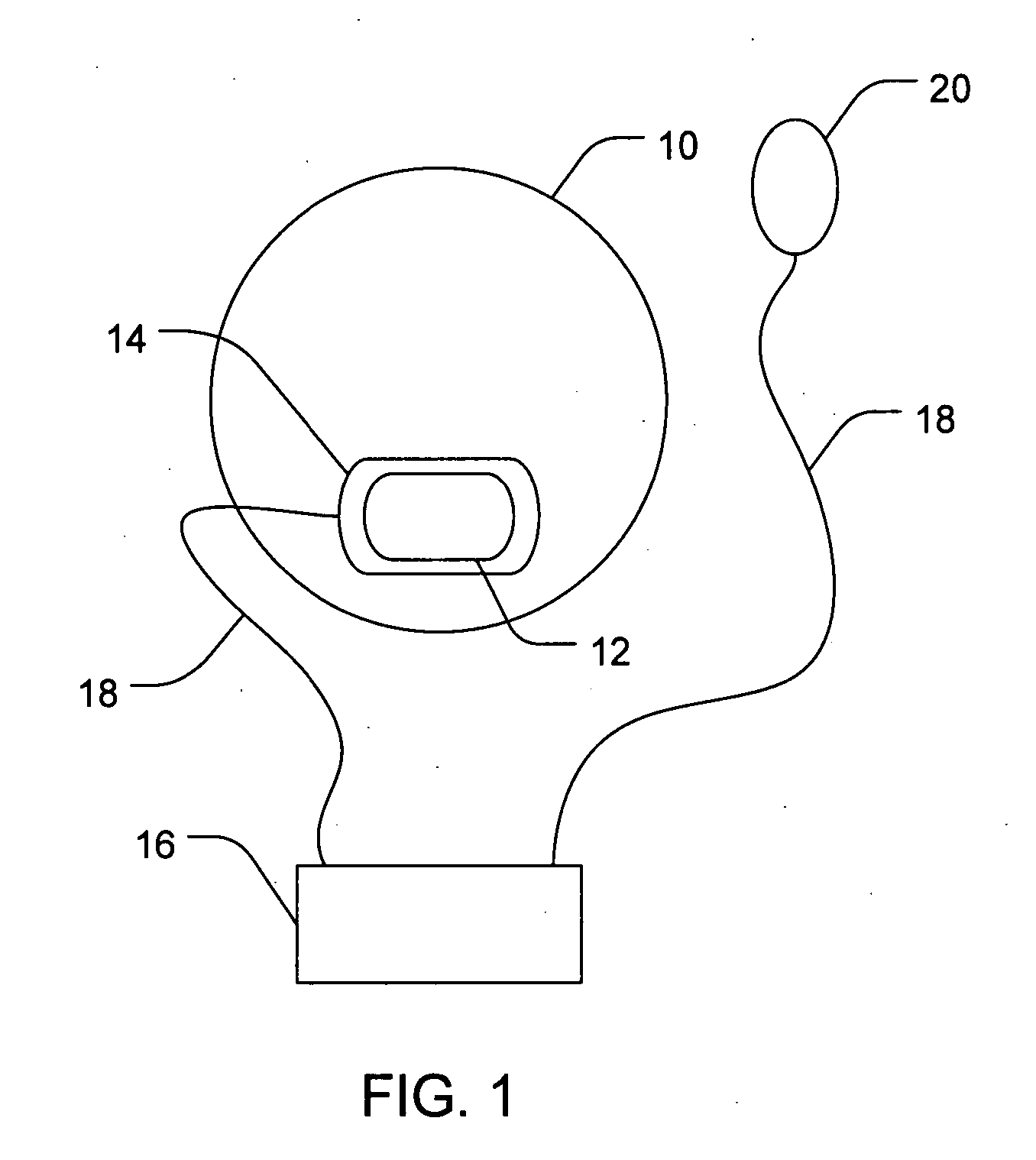
[0065] This example describes the non-invasive delivery of a triamcinolone acetonide agent into the eyes of rabbits. In this study, an ocular device was placed on the eyes of rabbits. The electrode chamber of the device was positioned on the conjunctiva near the pars plana. The triamcinolone acetonide agent in the electrode chamber was 0.5 M triamcinolone acetonide phosphate. The delivery of the active agent was achieved by applying a constant direct electric current of two milliampere across the electrode chamber for 15 minutes, thus delivering the triamcinolone acetonide agent. Six groups of 2 to 3 rabbits with each group assigned to the different time point (10-min, 4-hour, or 1-day) were used. At 10 minutes, 4 hours, and 1 day after the iontophoresis applications, the animals were euthanized and the eyes were enucleated for triamcinolone acetonide and triamcinolone acetonide phosphate assays. The assay procedure involved extracting these compounds from the conjunctiva, sclera, a...
example 2
[0066] This example describes the non-invasive delivery of a sustained release triamcinolone acetonide agent into the eyes of rabbits using a depot forming agent. In this study, an ocular device of side-by-side active and depot forming agent chambers was placed on the eyes of rabbits. The electrode chambers of the device were positioned on the conjunctiva near the pars plana. The active and depot forming agents were 0.5 M triamcinolone acetonide phosphate and 1.0 M dodecyl ammonium, respectively. The delivery of the sustained release system was achieved by applying a constant direct electric current of two milliampere across the side-by-side chambers for 15 minutes, in which the active agent was delivered from the cathode and the depot forming agent was from the anode. Six groups of 2 to 3 rabbits with each group assigned to the different time point (10-min, 4-hour, or 1-day) were used. At 10 minutes, 4 hours, and 1 day after the iontophoresis applications, the animals were euthaniz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com