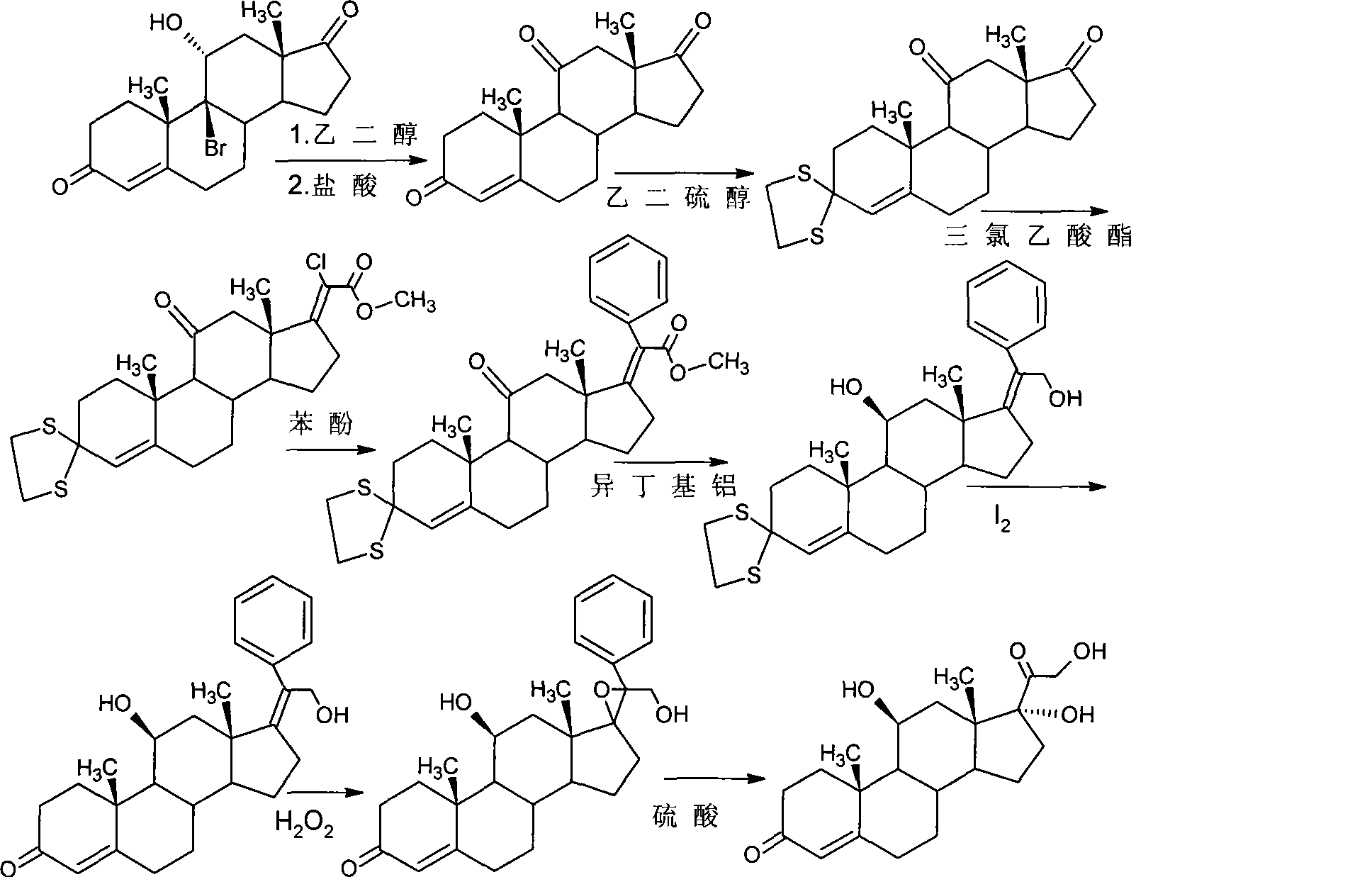
Preparation of hydrocortisone and derivatives thereof
A technology of hydrocortisone and compounds, which is applied in the field of preparation of steroid compounds, can solve the problems of low industrialization degree, high cost, and long route, and achieve the reduction of production cost and industrialization conditions, high feasibility, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
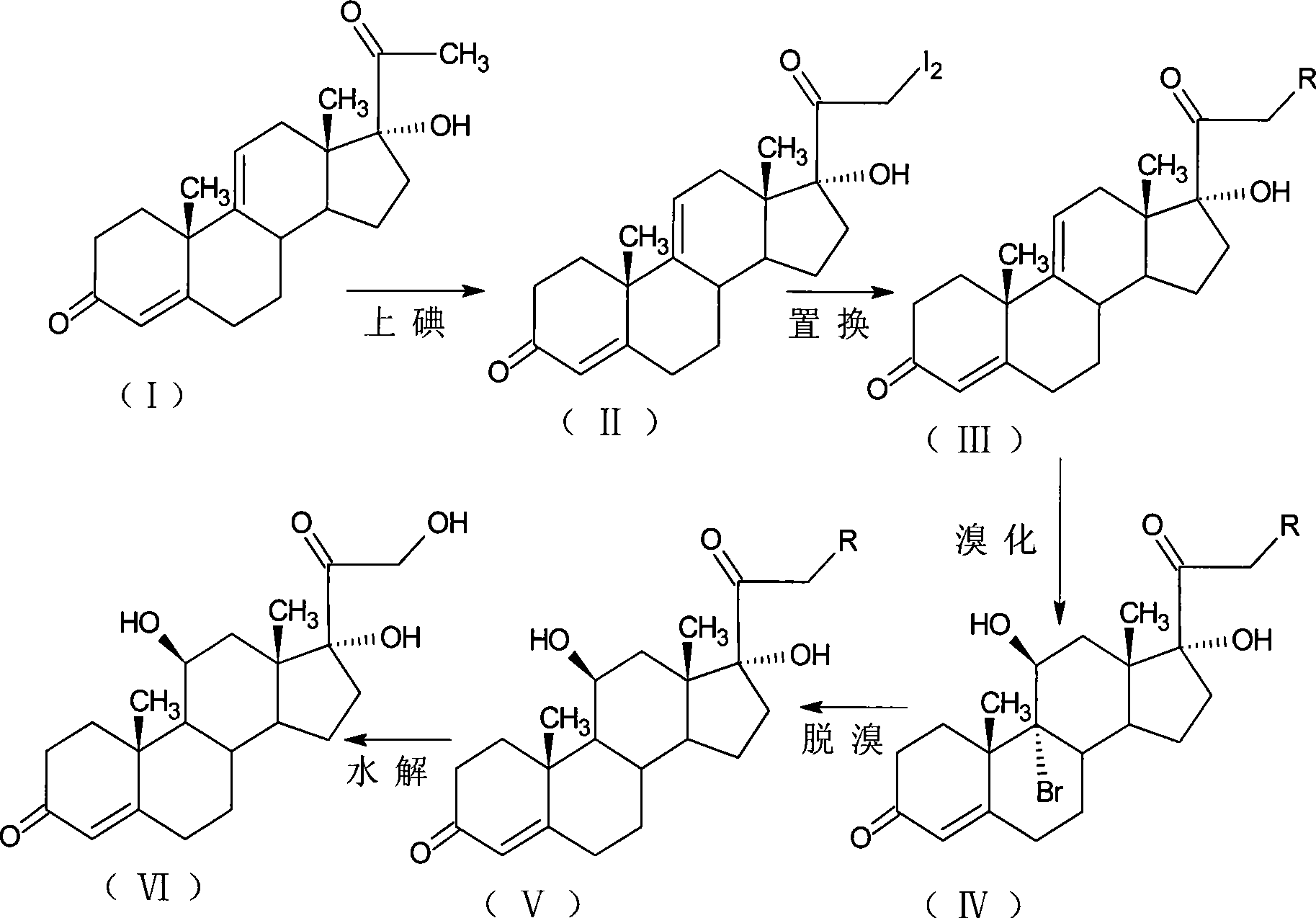
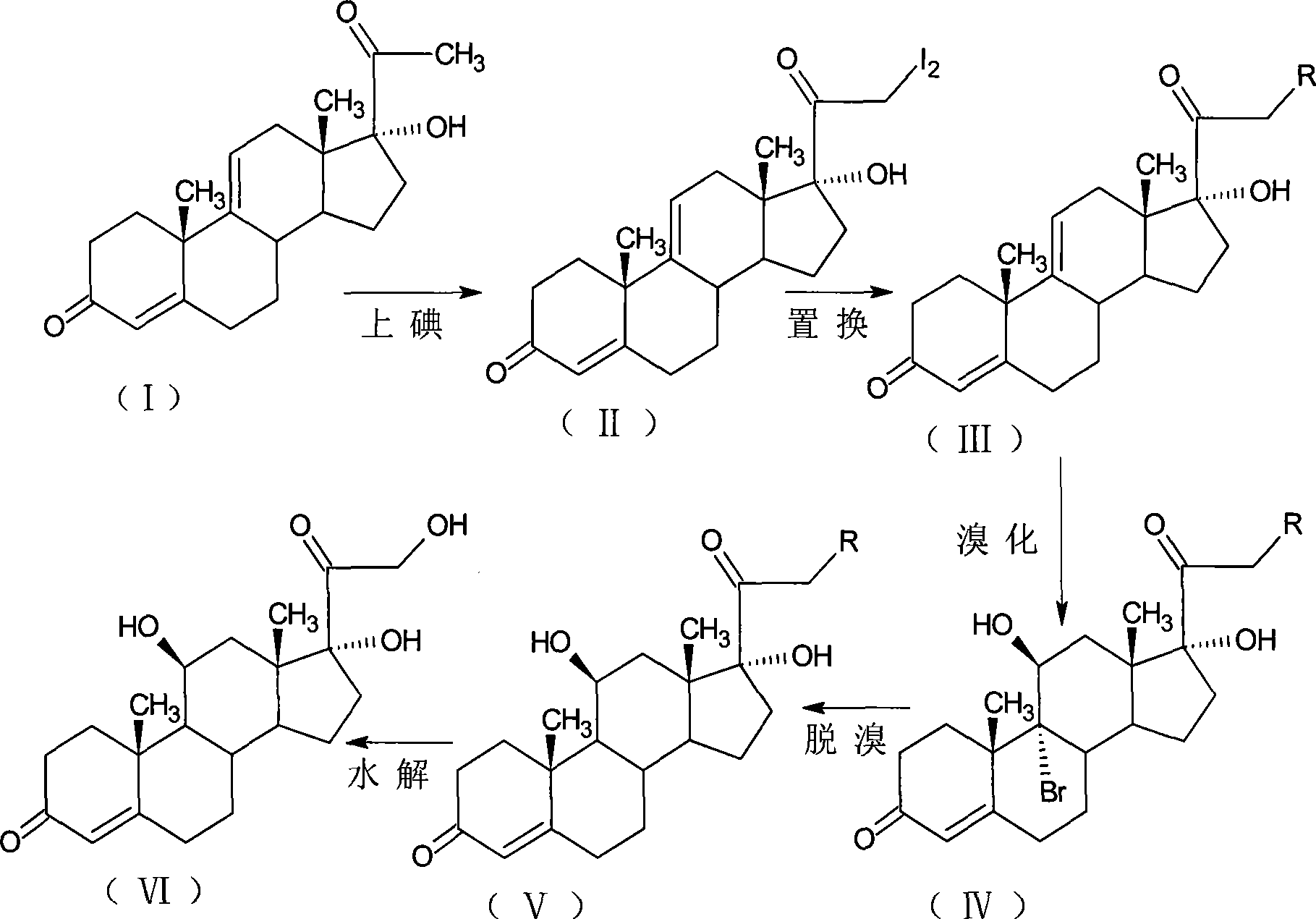
[0036] Iodine reaction: 21-diiodo-17α-hydroxy-4,9-diene-pregna-3,20-dione;
[0037] Add 150ml of methanol and 6g of calcium oxide to the reaction bottle, and 8.2g of anhydrous calcium chloride in 90ml of methanol solution in another volumetric flask, take out 1 / 4 after dissolving, add to the reaction bottle, and dissolve the rest 15g of iodine particles, Add 10g of 17α-hydroxy-4,9-diene-pregna-3,20-dione (CN1896090), fill with nitrogen gas, control the temperature at 0±5°C, add iodine solution dropwise, and drop it in about 3 hours. After another 1 hour of reaction, the reaction solution was diluted in 600ml of 2% ammonium chloride aqueous solution, stirred for 1 hour, left to stand for 1 hour, filtered, washed with water to neutrality, and obtained wet product iodide (II). It is stable and does not need to be dried. It should not be placed for too long. It is ready for use.
[0038] Replacement reaction: 17α,21-dihydroxy 4,9-diene-pregna-3,20-dione-21-acetate
[0039] Add 5...
Embodiment 2
[0047] Iodine reaction: 21-diiodo-17α-hydroxy-4,9-diene-pregna-3,20-dione;
[0048] Add 50ml of tetrahydrofuran and 6g of calcium oxide to the reaction flask, and 8.2g of anhydrous calcium chloride in 90ml of methanol solution in another volumetric flask, take out 1 / 4 after dissolving, add to the reaction flask, and dissolve the rest 15g of iodine particles, Add 10g of 17α-hydroxy-4,9-diene-pregna-3,20-dione (CN1896090), fill with nitrogen gas, control the temperature at 0±5°C, add iodine solution dropwise, and drop it in about 3 hours. After another 1 hour of reaction, the reaction solution was diluted in 600ml of 2% ammonium chloride aqueous solution, stirred for 1 hour, left to stand for 1 hour, filtered, washed with water to neutrality, and obtained wet product iodide (II). It is stable and does not need to be dried. It should not be placed for too long. It is ready for use.
[0049] Displacement reaction: 17α,21-dihydroxy-4,9-diene-pregna-3,20-dione-21-propionate
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com