Mesalazine-containing drug composition
A composition and drug technology, applied in the field of pharmaceutical compositions containing mesalazine, can solve the problems of affecting the therapeutic effect, failure, and easy to be oxidized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Prescription (specification: 60g:4g):
[0013]
[0014] Preparation Process:
[0015] Take prescription amount of calcium sodium edetate, sodium acetate, sodium benzoate, add appropriate amount of purified water and stir to dissolve, under stirring, add prescription amount of xanthan gum and carbomer, stir to swell, add prescription amount of bisulfite Sodium and mesalazine, stir and mix well, homogenize, add water to the full amount, stir evenly, and fill it. The measured PH value is 3.5, and the measured particle size is 90%. The particle size ranges from 8 microns to 15 microns.
Embodiment 2
[0017] Prescription (specification: 60g:4g):
[0018]
[0019] Preparation Process:
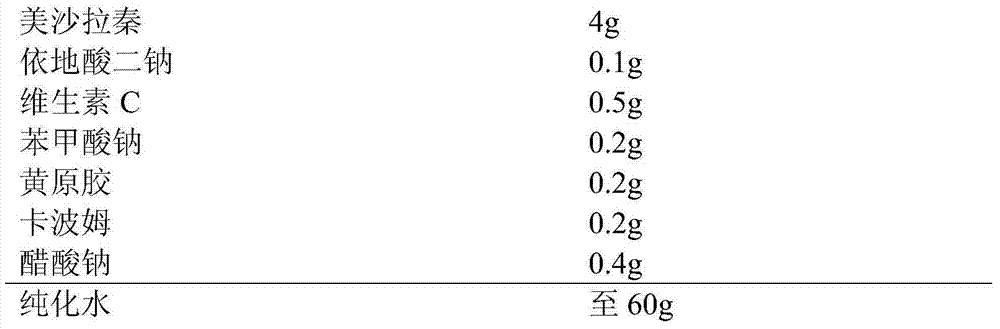
[0020] Take prescription amount of disodium edetate, sodium acetate, sodium benzoate, add appropriate amount of purified water and stir to dissolve, under stirring, add prescription amount of xanthan gum and carbomer, stir to swell, add prescription amount of vitamin C, Mesalazine, stir and mix well, homogenize, add water to the full amount, stir evenly, and fill it. The measured PH value is 5.5, and the measured particle size is 90%. The particle size ranges from 8 microns to 15 microns.
Embodiment 3
[0022] Prescription (specification: 60g:4g):
[0023]
[0024] Preparation Process:
[0025] Take prescription amount of disodium edetate, sodium acetate, sodium benzoate, add appropriate amount of purified water and stir to dissolve, under stirring, add prescription amount of xanthan gum and carbomer, stir and swell, add prescription amount of sodium metabisulfite, Mesalazine, stir and mix well, homogenize, add water to the full amount, stir evenly, and fill it. The measured PH value is 4.8, and the measured particle size is 90%. The particle size ranges from 8 microns to 15 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com