Method for producing biologically ingestible microparticles, biologically ingestible microparticles, and dispersion and pharmaceutical composition containing the same
a technology of biological ingestible microparticles and biological ingestible microparticles, which is applied in the direction of drug compositions, peptide/protein ingredients, immunological disorders, etc., can solve the problems of increasing the surface area of the formulation of drugs and medicines, increasing the processing time, and increasing the surface area. , to achieve the effect of reducing processing time, high production efficiency and high biological availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Danazol Particles
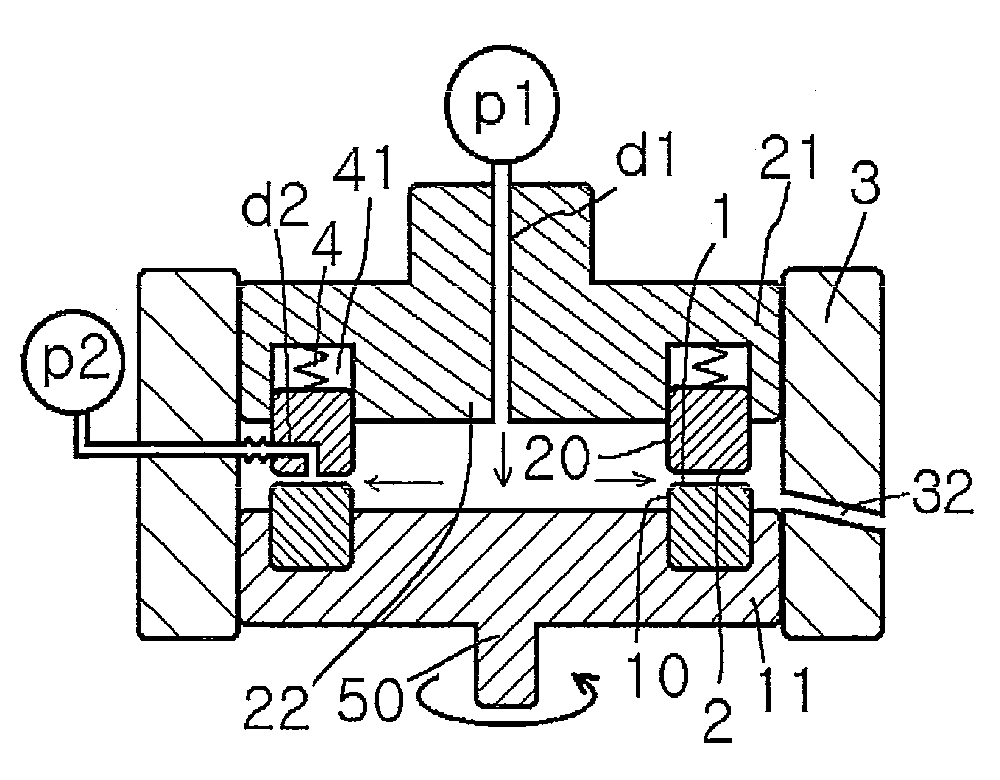
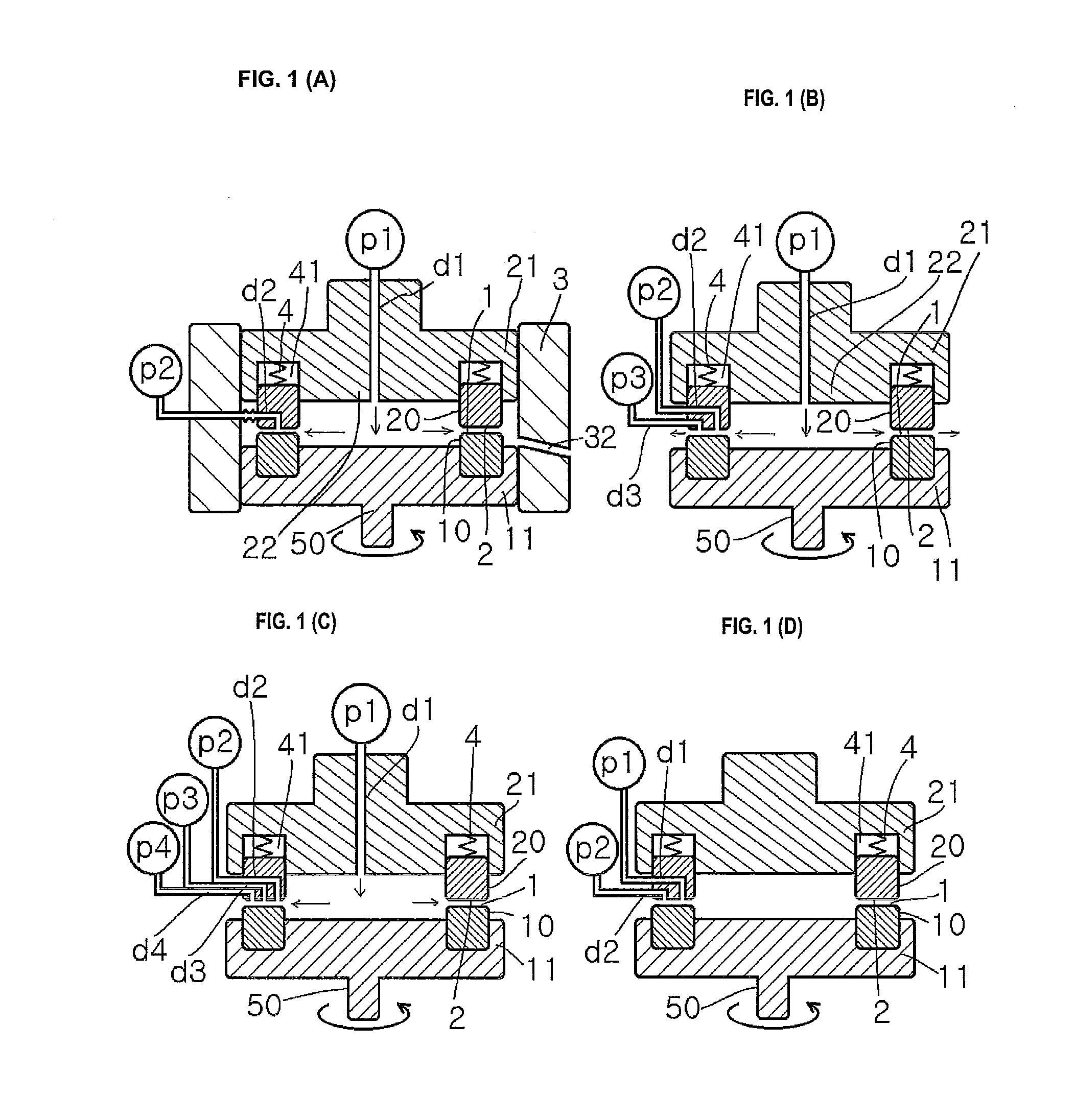
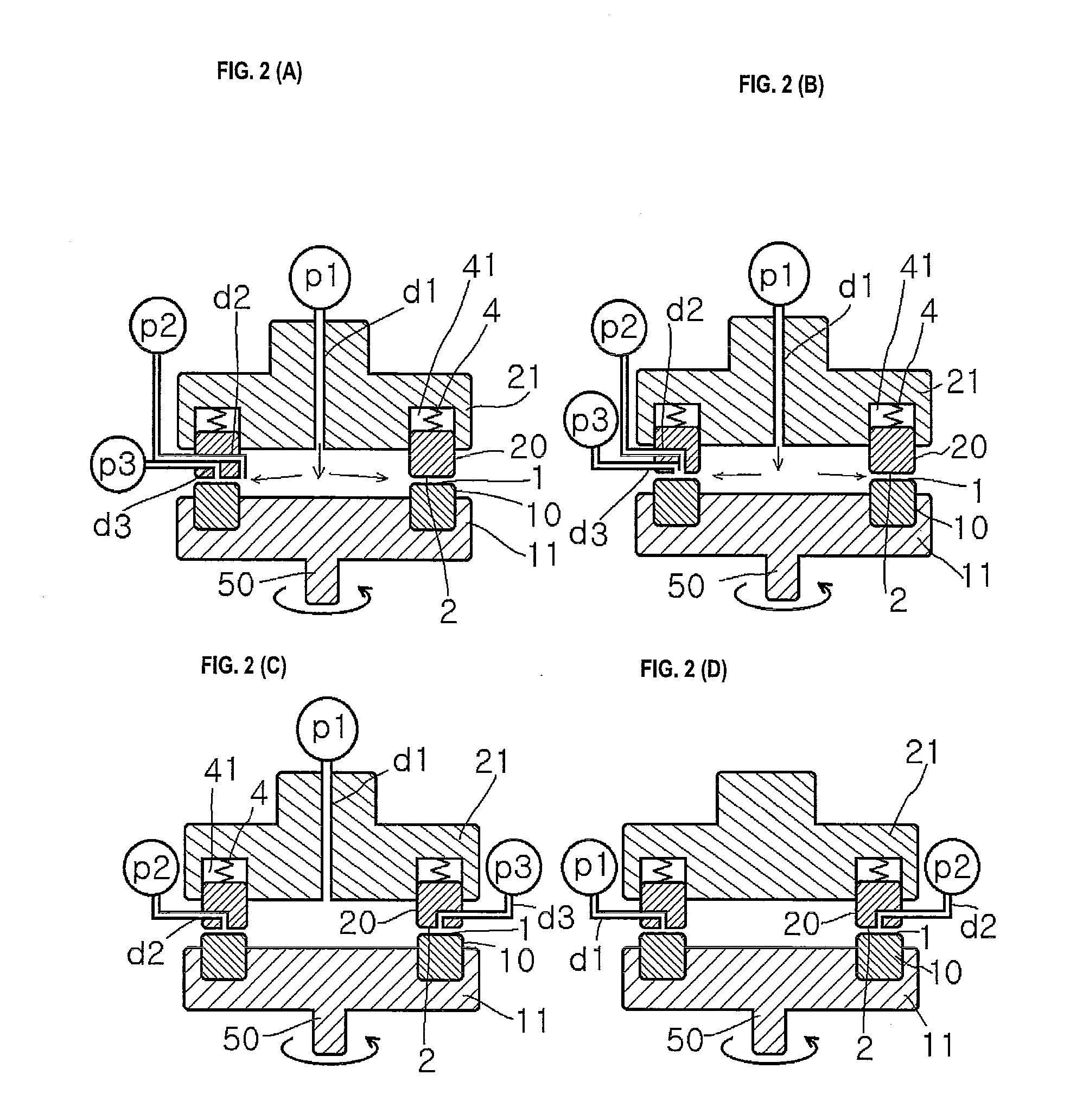
[0453]A mixed solution of an aqueous solution having the drug dissolved therein, a solution having low solubility for the drug, and a polymer dispersant or a stabilizer is subjected to crystallization reaction in a thin film fluid formed between the processing surfaces 1 and 2 arranged to be opposite to each other so as to be able to approach to and separate from each other, at least one of which rotates relative to the other, in the uniformly dispersing, stirring and mixing reaction apparatus shown in FIG. 1(A).
[0454]While 0.1% aqueous solution of Tween 80 was sent as a first fluid from the center at a supply pressure / back pressure of 0.02 MPa / 0.01 MPa, at a revolution number of 1000 rpm and at a sending solution temperature of 35° C., a solution prepared by dissolving powdery danazol in ethanol was introduced at a rate of 10 ml / min. as a second fluid into the space between the processing surfaces. The first and second fluids were mixed with each othe...
example 2
Production of Tacrolimus Hydrate Particles
[0465]While 0.1% aqueous solution of Tween 80 was sent as a first fluid from the center at a supply pressure / back pressure of 0.02 MPa / 0.01 MPa, at a revolution number of 2000 rpm and at a sending solution temperature of 30° C., a solution prepared by dissolving tacrolimus hydrate in ethanol was introduced at a rate of 10 ml / min. as a second fluid into the space between the processing surfaces. The first and second fluids were mixed with each other in the thin film, and a tacrolimus hydrate dispersion solution was discharged at a rate of 30 g / min. from the processing surfaces.
[0466]When the recovered tacrolimus hydrate dispersion solution was measured with a particle size distribution measuring instrument utilizing a dynamic light scattering method as measurement principle (trade name: Microtrac UPA150, manufactured by Nikkiso Co., Ltd.), the (volume) average particle size was 116 nm and the CV value of its particle size distribution / particl...
example 3
Production of Tranilast Particles
[0471]While water was sent as a first fluid from the center at a supply pressure / back pressure of 0.02 MPa / 0.01 MPa, at a revolution number of 1000 rpm and at a sending solution temperature of 27° C., a solution prepared by dissolving tranilast in a Tween 80-containing potassium hydroxide solution, pH 13 was introduced at a rate of 10 ml / min. as a second fluid into the space between the processing surfaces. The first and second fluids were mixed with each other in the thin film, and a tranilast dispersion solution was discharged at a rate of 30 g / min. from the processing surfaces.
[0472]When the recovered tranilast hydrate dispersion solution was measured with a particle size distribution measuring instrument utilizing a dynamic light scattering method as measurement principle (trade name: Microtrac UPA150, manufactured by Nikkiso Co., Ltd.), the (volume) average particle size was 120 nm and the CV value of its particle size distribution / particle diam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size distribution/particle diameter distribution | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com