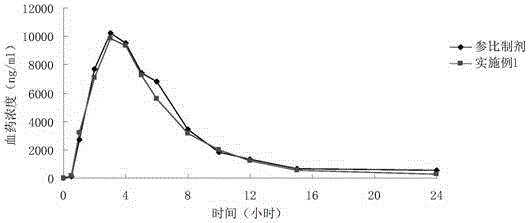
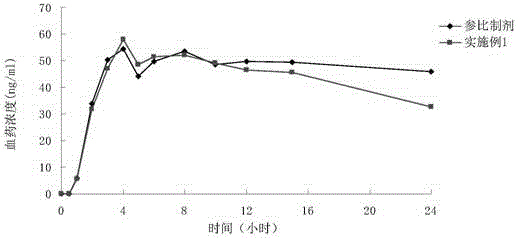
Mesalazine enteric-coated sustained-release pellet and preparation method thereof
A technology of mesalazine and sustained-release pellets, which can be applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems such as the inability to obtain a uniform sustained-release film and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1) Sustained release pill core
[0055]
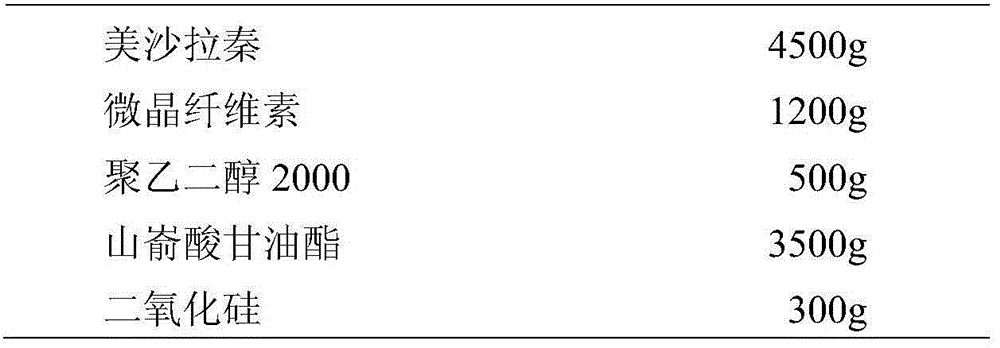
[0056] Mix mesalamine with microcrystalline cellulose, glyceryl behenate, silicon dioxide, and polyethylene glycol evenly, add water to moisten evenly, extrude into 1mm strip soft material with an extrusion spheronizer, and spheroidize to about 1mm spherical wet pills. The wet pellets were dried at about 60°C. After drying, sieve the sustained-release pellet cores with sieves with apertures of 0.5 mm and 1 mm.
[0057] 2) Enteric coating
[0058]
Embodiment 2
[0061] 1) Sustained release pill core
[0062]
[0063] Mix mesalazine with microcrystalline cellulose, glyceryl behenate, and polymerized sugar evenly, add water to moisten evenly, use an extrusion spheronizer to extrude into a strip-shaped soft material of 1mm, and spheroidize into a spherical wet pellet of about 1mm. The wet pellets were dried at about 60°C. After drying, sieve the sustained-release pellet cores with sieves with apertures of 0.5 mm and 1 mm.
[0064] 2) Isolation coating
[0065] Easy-release premix with film coating TM The sustained-release ball core is coated with an isolation coat, and the weight of the coating is increased by 5%.
[0066] 3) Enteric coating
[0067]
[0068] Eudragit L100 and S100 were dissolved in 1500g 80% ethanol solution, and then talcum powder and triethyl citrate were dispersed into the solution; the above-mentioned enteric coating solution was sprayed in the fluidized bed (bottom spray) coating equipment On gown beads....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com