Doxorubicin hydrochloride light-controlled sustained-release liquid crystal gel preparation and preparation method thereof
A technology for doxorubicin hydrochloride and gel preparations, which is applied in the field of doxorubicin hydrochloride light-controlled sustained-release liquid crystal gel preparations and its preparation, achieving the effects of reducing the number of medications, easy industrialization, and low equipment and raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A light-controlled sustained-release liquid crystal gel preparation of doxorubicin hydrochloride, which is prepared from the following raw materials in percentage by weight: 25.99% phosphatidylcholine, 60% glyceryl dioleate, 13.88% absolute ethanol, and doxorubicin hydrochloride 0.1%, IR8200.03%.
[0038] The preparation method of the doxorubicin hydrochloride light-controlled slow-release liquid crystal gel preparation is as follows: after mixing phosphatidylcholine, diolein glyceride, doxorubicin hydrochloride and IR820 according to the weight percentage, then add the The absolute ethanol in the weight percentage is mixed evenly on a mixer to make it completely dissolved, and the doxorubicin hydrochloride light-controlled sustained-release liquid crystal gel preparation is obtained, which is sealed and stored at 4°C.
[0039] The appearance of the product obtained in this example is a light yellow transparent solution, which shows that each raw material has good compa...
Embodiment 2
[0042] A doxorubicin hydrochloride light-controlled slow-release liquid crystal gel preparation, prepared from the following raw materials in weight percentage: 35% phosphatidylcholine, 52% glyceryl dioleate, 12.12% absolute ethanol, doxorubicin hydrochloride 0.8%, IR8200.08%.
[0043] The preparation method is the same as in Example 1.
[0044] The appearance of the product obtained in this example is a light yellow transparent solution, which shows that each raw material has good compatibility and is evenly mixed. There is no delamination phenomenon after storage at -4°C for 6 months and centrifugation treatment, indicating that the product has good storage stability.
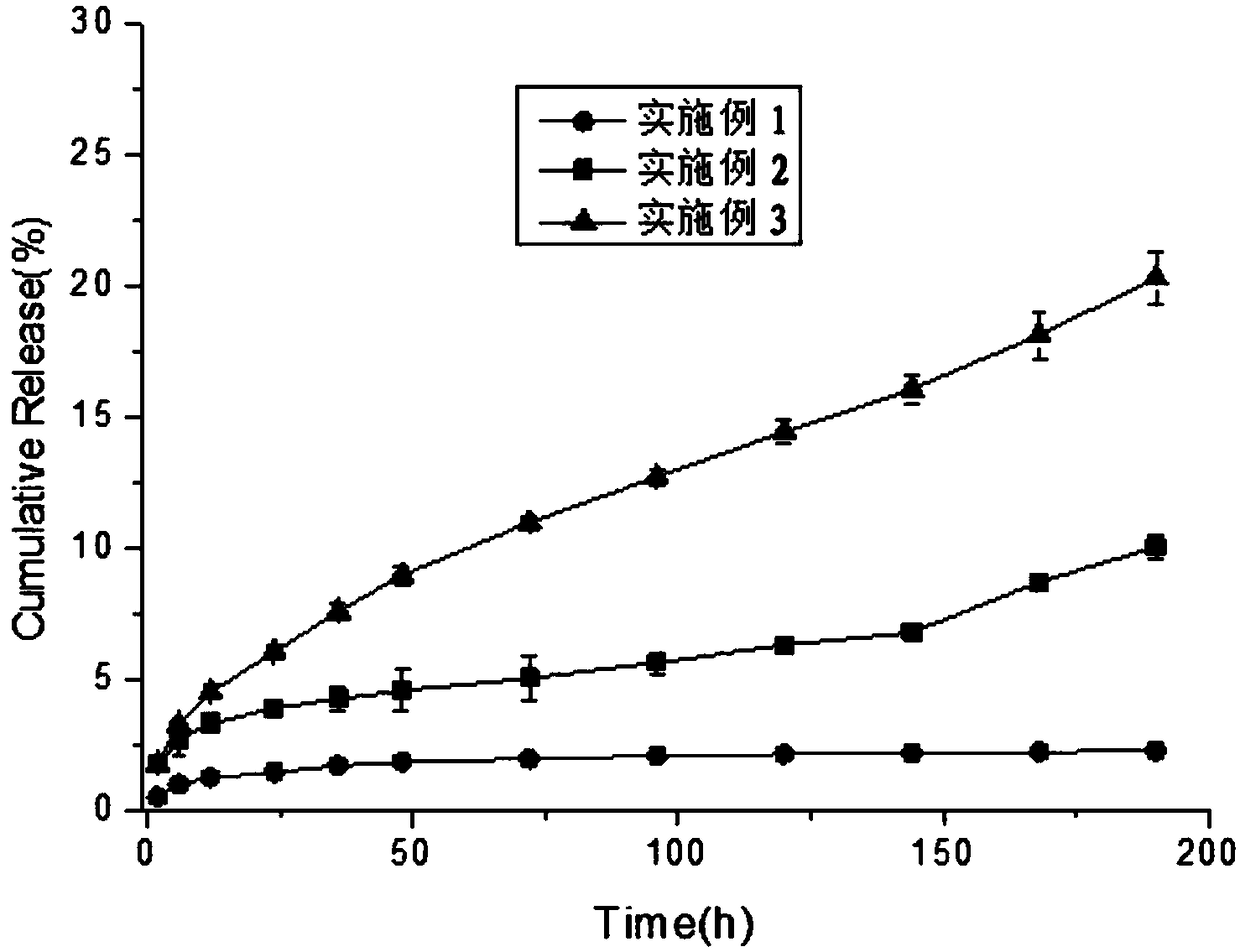
[0045] In this example, the cumulative release rate of doxorubicin hydrochloride in the light-controlled sustained-release liquid crystal gel preparation precursor of doxorubicin hydrochloride within one week without and with light were 10.05±0.43% and 22.25±0.21%, respectively. The results are as follows ...
Embodiment 3
[0047] A light-controlled sustained-release liquid crystal gel preparation of doxorubicin hydrochloride, which is prepared from the following raw materials in percentage by weight: phosphatidylcholine 25%, glyceryl dioleate 54.45%, absolute ethanol 20%, doxorubicin hydrochloride 0.5%, IR8200.05%.
[0048] The preparation method is the same as in Example 1.
[0049] The appearance of the product obtained in this example is a light yellow transparent solution, which shows that each raw material has good compatibility and is evenly mixed. There is no delamination phenomenon after storage at -4°C for 6 months and centrifugation treatment, indicating that the product has good storage stability.
[0050] In this embodiment, the cumulative release rate of doxorubicin hydrochloride in the precursor of doxorubicin hydrochloride light-controlled slow-release liquid crystal gel preparation is about 21.15±0.18% within a week without and with light, and the results are as follows image ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com