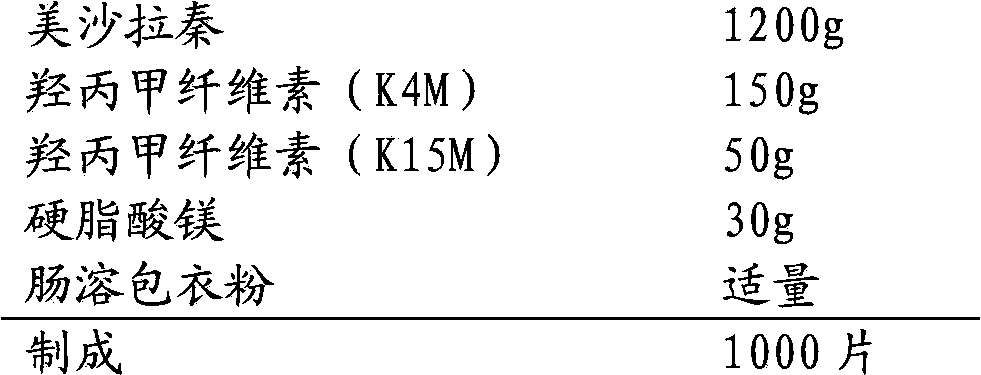Mesalazine enteric-coated sustained release tablet
A technology of mesalazine and sustained-release tablets, which is applied in the direction of pill delivery, digestive system, organic active ingredients, etc., and can solve problems such as changes in related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
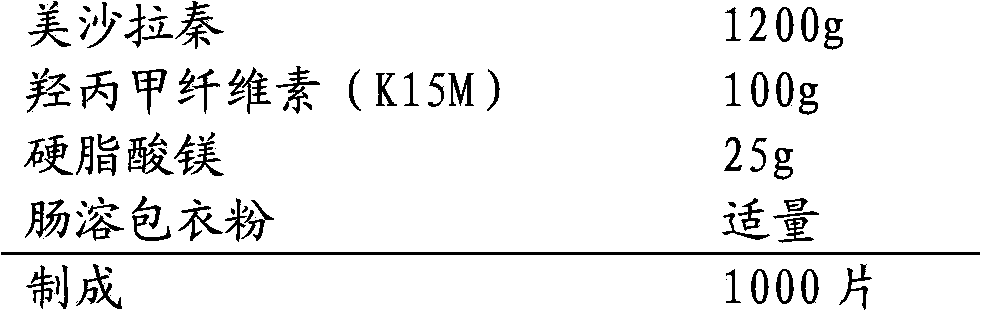
[0019] 1. Prescription:
[0020]
[0021] 2. Process steps:
[0022] (1) Hypromellose (K15M) and mesalazine are mixed evenly, then part of the lubricant is added and mixed to obtain a mixed powder;
[0023] (2) The compressed tablet of mixed powder has a diameter of 7mm and a hardness of 4KG. Then the tablets are crushed through an 18-mesh sieve to obtain granules;
[0024] (3) The obtained granules are mixed with the remaining lubricant, and the tablets are compressed. The tablets are elliptical in shape and the long-side hardness is 14-17KG;
[0025] (4) The obtained tablets are coated with enteric coating powder.
Embodiment 2
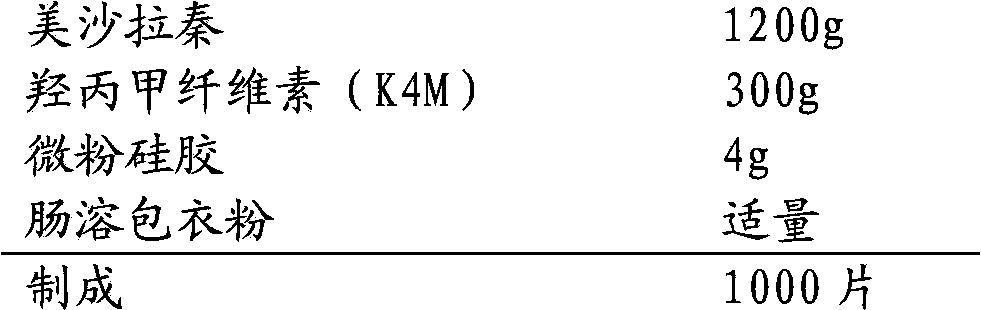
[0027] 1. Prescription:
[0028]
[0029] 2. Process steps:
[0030] (1) Hypromellose (K4M) and mesalazine are mixed evenly, and then part of the lubricant is added and mixed to obtain a mixed powder;
[0031] (2) The compressed tablet of mixed powder has a diameter of 10mm and a hardness of 8KG. Then the tablets are crushed through a 14-mesh sieve to obtain granules;
[0032] (3) The obtained granules are mixed with the remaining lubricant, and the tablets are compressed. The tablets are elliptical in shape and the long-side hardness is 14-17KG;
[0033] (4) The obtained tablets are coated with enteric coating powder.
Embodiment 3
[0035] 1. Prescription:
[0036]
[0037] 2. Process steps:
[0038] (1) Hypromellose (K4M), hypromellose (K15M) and mesalazine are mixed uniformly, and then part of the lubricant is mixed to obtain a mixed powder;
[0039] (2) The compressed tablet of mixed powder has a diameter of 8mm and a hardness of 6KG. Then the tablets are crushed through an 18-mesh sieve to obtain granules;
[0040] (3) The obtained granules are mixed with the remaining lubricant, and the tablets are compressed. The tablets are elliptical in shape and the long-side hardness is 14-17KG;
[0041] (4) The obtained tablets are coated with enteric coating powder.
[0042] Release test
[0043] Release conditions: simulated release in pH7.2 intestinal juice, using 900ml of pH7.2 sodium phosphate buffer, paddle method, rotation speed, 50r / min, temperature: 36.5-37.5℃.
[0044] Samples were taken at 1 hour, 4 hours, and 8 hours to determine the release degree. The results are as follows:
[0045]
[0046] Stability test
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com