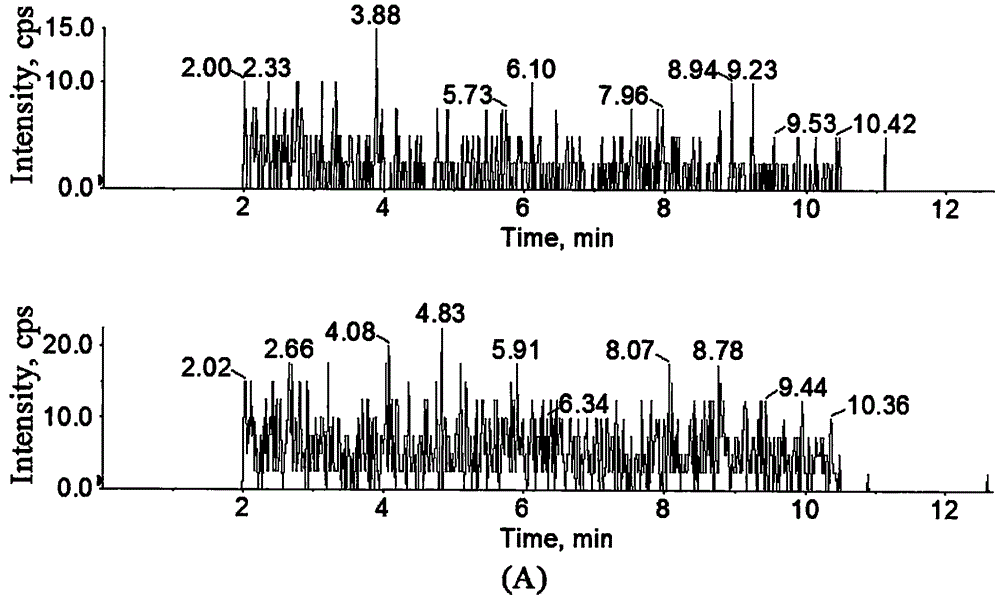
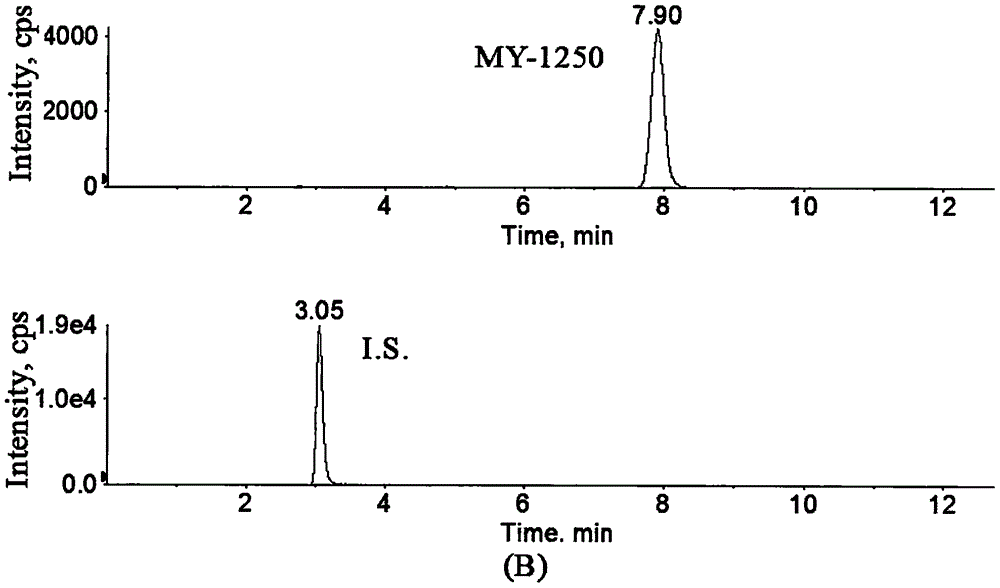
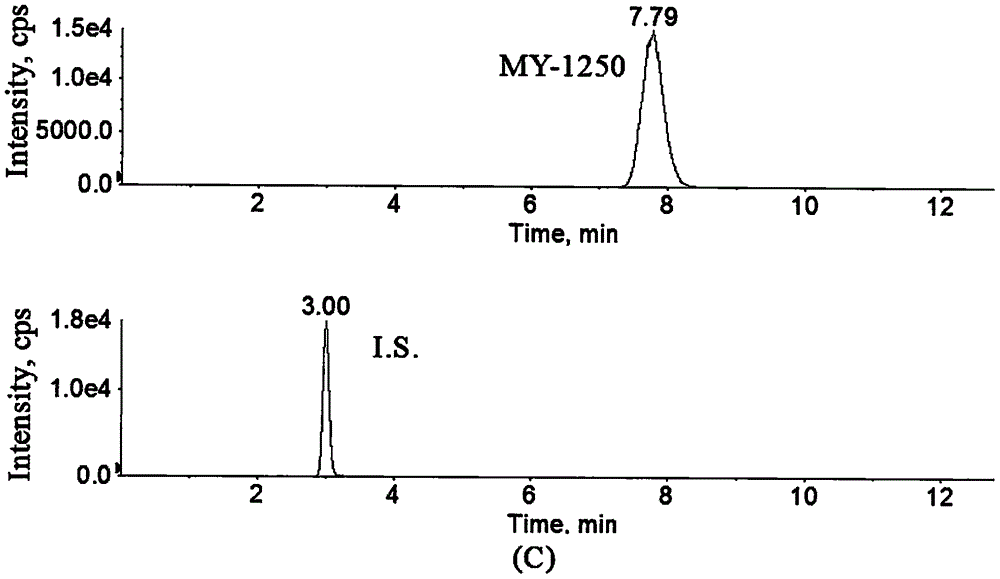
Method for determination of concentration of 5, 6-dihydro-7,8-dimethyl-4,5-dioxy-4-H-pyranoquinoline-2-carboxylic acid in plasma
A pyranquinoline and dimethyl technology, which is applied in the field of drug analysis, can solve the problem that the determination method is not reported in literature and the like, and achieve the effects of simple pretreatment method, high sensitivity and rapid method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Determination of MY-1250 concentration in human plasma.
[0028] 1. Experimental materials and instruments
[0029] MY-1250 reference substance: provided by Henan Furentang Pharmaceutical Co., Ltd., batch number: 20140501D; levetiracetam reference substance: provided by Zhejiang Huahai Pharmaceutical Co., Ltd., batch number: 201308-2; test water: ultrapure water Methanol: chromatographically pure (Merck Company); ammonia water, ammonium acetate: analytically pure (Sinopharm Chemical Reagent Co., Ltd.); hydrochloric acid: analytically pure (Shanghai Lingfeng Chemical Reagent Co., Ltd.).
[0030] API 4000 LC / MS / MS coupled instrument (Applied Biosystems, USA), chromatographic workstation: Analyst 1.6; Mettler XS 105DU electronic balance (Swiss Mettler company); Eppendorf Centrifuge 5424R high-speed low-temperature centrifuge (German Eppendorf company ); KDC-2042 low-speed refrigerated centrifuge (Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd.); Millipor...
Embodiment 2
[0052] Example 2: Determination of MY-1250 concentration in human plasma
[0053] Referring to Example 1, 5 healthy female subjects took 150 mg of Repimilast Tablets on an empty stomach, and took them with 250 mL of warm water; 4 hours after the administration, 3 mL of peripheral venous blood was collected, injected into a heparin tube, and centrifuged (4000 r min -1 , 5min), draw 600μL of subject’s plasma sample, add 12μL of hydrochloric acid solution (5mol·L -1 ), vortexed to prepare plasma. The treatment and measurement conditions of the drug-containing plasma samples were the same, and the results showed that the MY-1250 contents in the plasma of 5 healthy female subjects after oral administration of 150 mg of Repimilast Tablets on an empty stomach were 50.24, 52.71, 41.2, 26.12, and 43.45 ng·mL, respectively. -1 .
Embodiment 3
[0054] Example 3: Determination of MY-1250 Concentration in Human Plasma
[0055] Referring to Example 1, 5 healthy male subjects took 50 mg / bag × 3 bags of Repimilast Granules on an empty stomach, and took it with 250 mL of warm water; 2 hours after administration, 3 mL of peripheral venous blood was collected, injected into a heparin tube, and centrifuged (4000 r min -1 , 5min), draw 600μL of subject’s plasma sample, add 12μL of hydrochloric acid solution (5mol·L -1 ), vortexed to prepare plasma. The treatment and measurement conditions of the drug-containing plasma samples were the same, and the results showed that the contents of MY-1250 in the plasma of 5 healthy male subjects after oral administration of 150 mg of Repimilast Granules on an empty stomach were 49.26, 107.6, 56.76, 100.7, and 124.5 ng mL respectively. -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com