Method for determining mesalazine related substances by high performance liquid chromatography
A detection method and mesalazine technology, applied in the field of medicine, can solve the problems of easy splitting of peaks, poor peak shape, and unsatisfactory resolution, and achieve the effect of ensuring drug safety and controllability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further described in detail below in conjunction with the examples.
[0025] The following examples can help those skilled in the art to understand the present invention more comprehensively, but do not limit the present invention in any way.
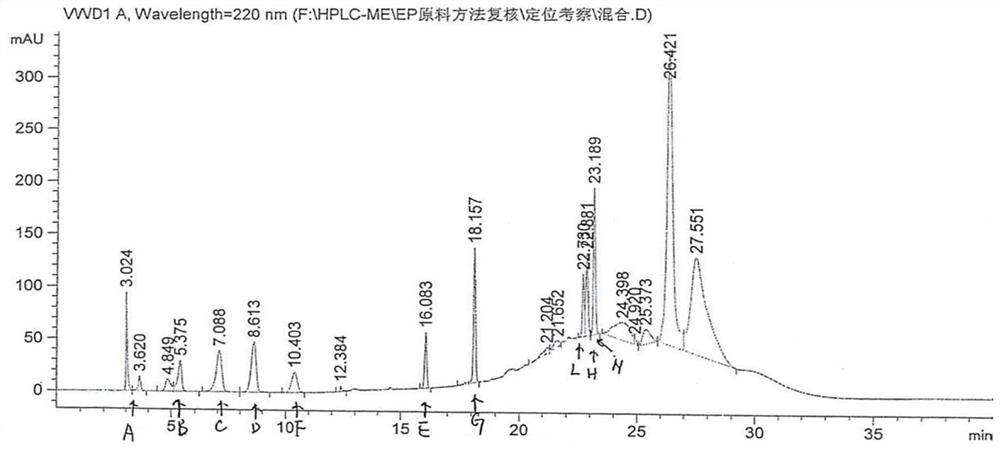
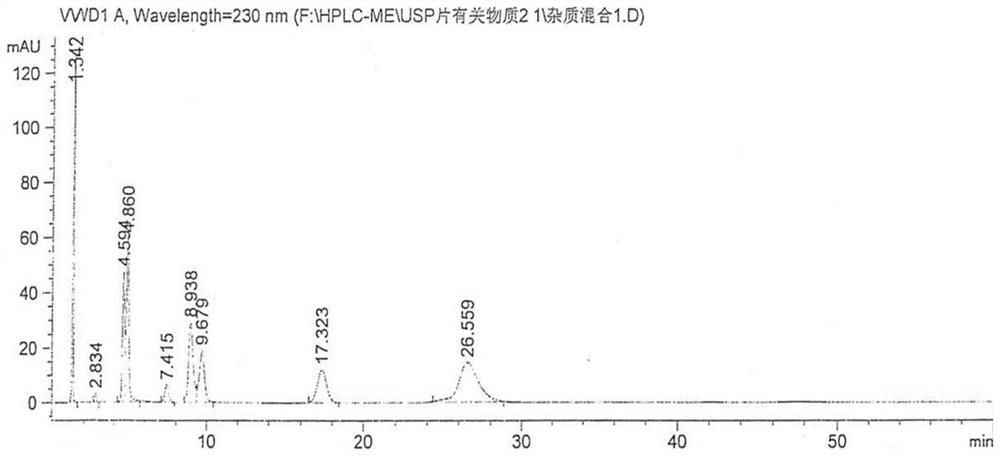
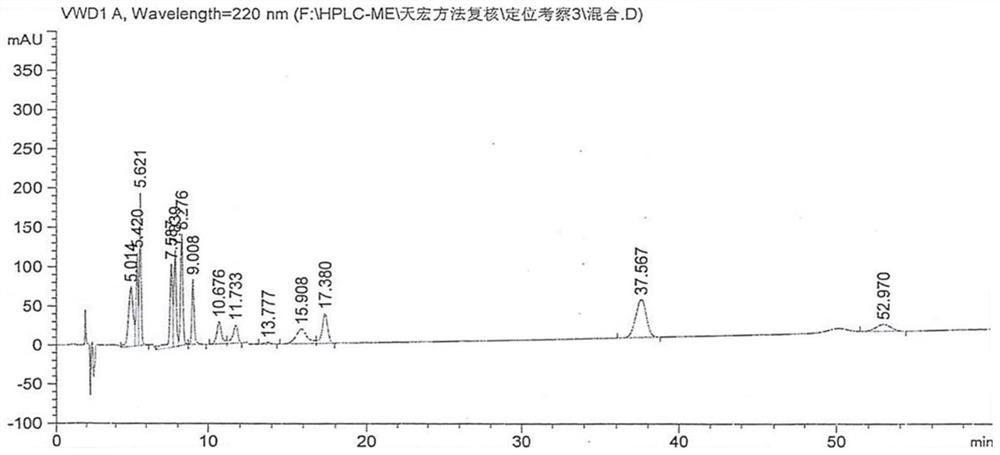
[0026] Condition 1: Mesalazine API EP (related substance system 1)
[0027] Use the column as C 8 Column (250×4.6nm, 5μm), using gradient elution method: mobile phase A (take 2.2g perchloric acid and 1.0g phosphoric acid, dilute with water to 1000ml); mobile phase B (take 1.7g perchloric acid and 1.0g Phosphoric acid, diluted to 1000ml with acetonitrile), the detection wavelength is 220nm, the flow rate is 1.25ml / min, and the injection volume is 10μl.
[0028] The gradient elution method is as follows:
[0029]
[0030] Mesalazine positioning solution: Take 5 mg of mesalamine, weigh it accurately, put it in a 10ml measuring bottle, add an appropriate amount of 50% methanol, sonicate for 10 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com