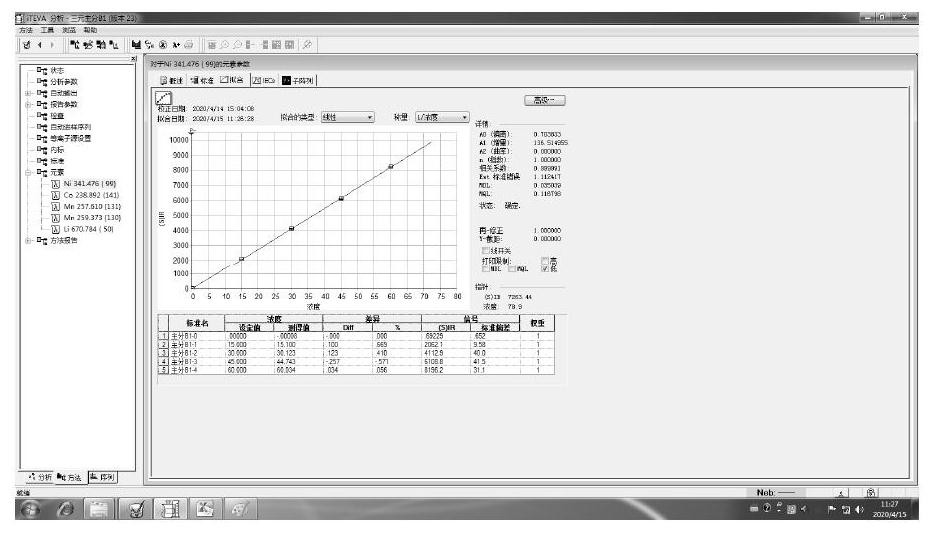
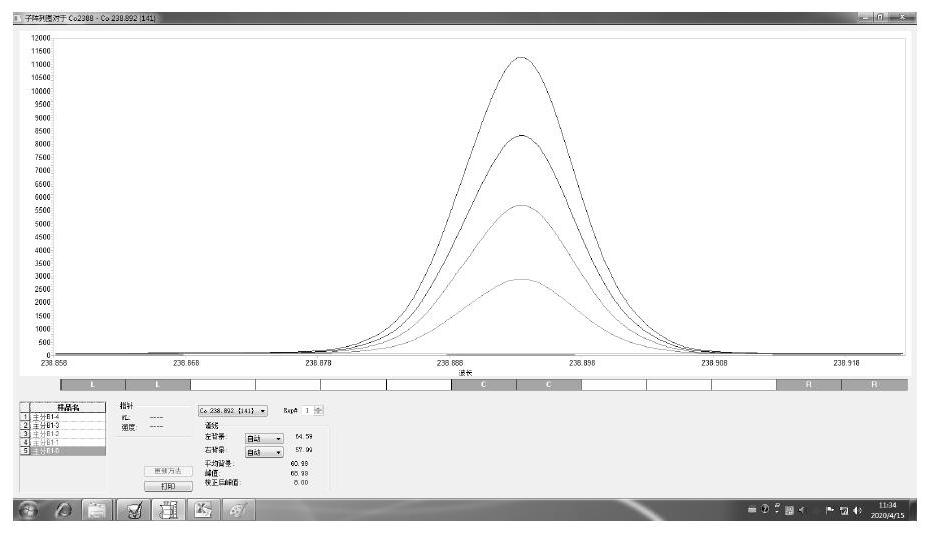
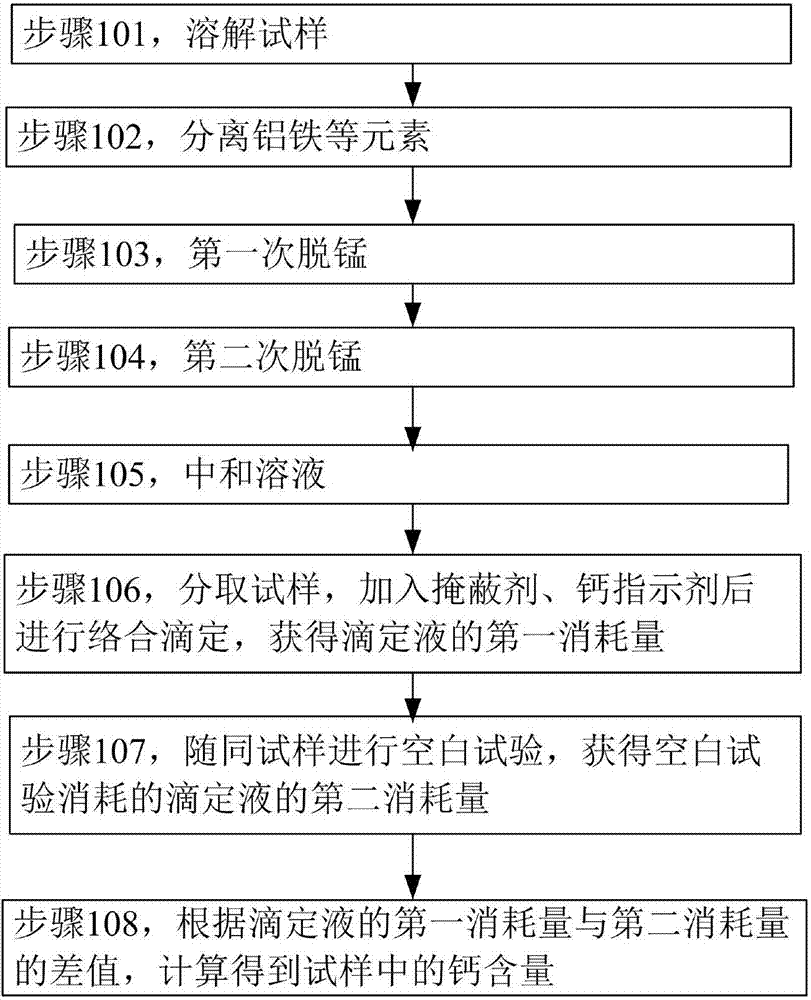
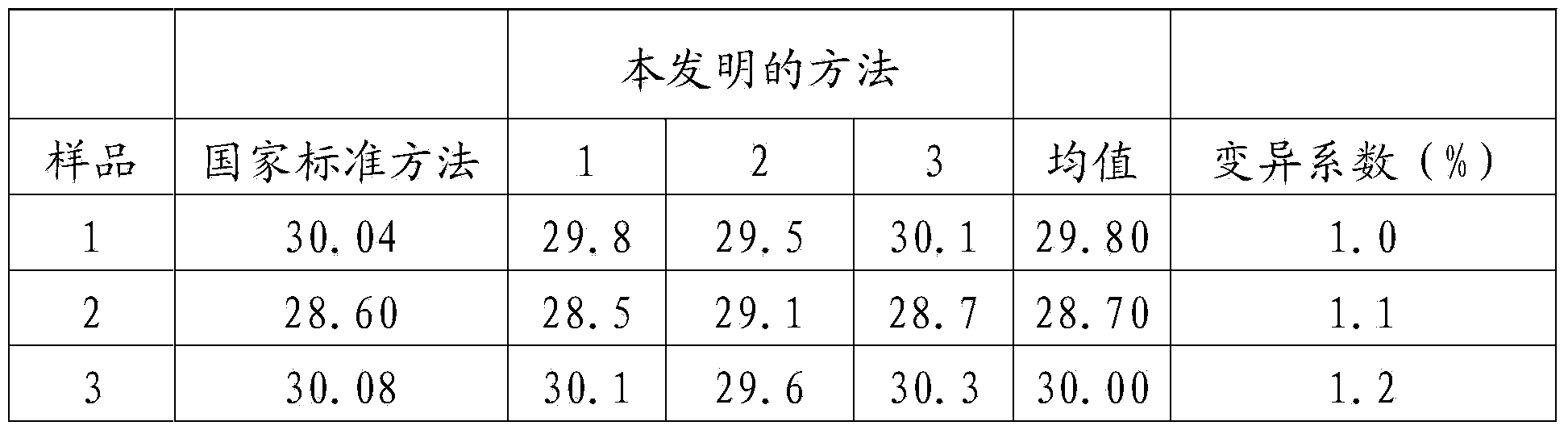
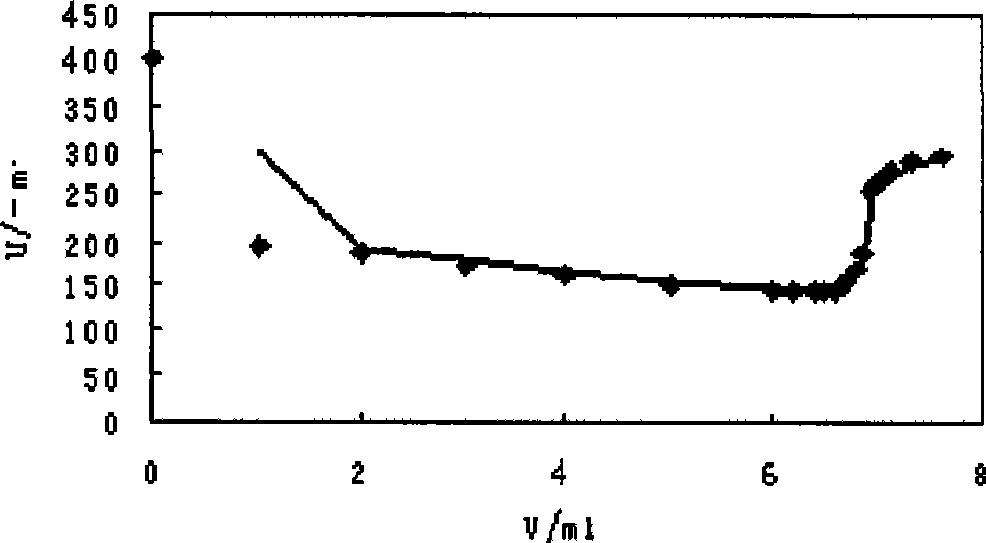
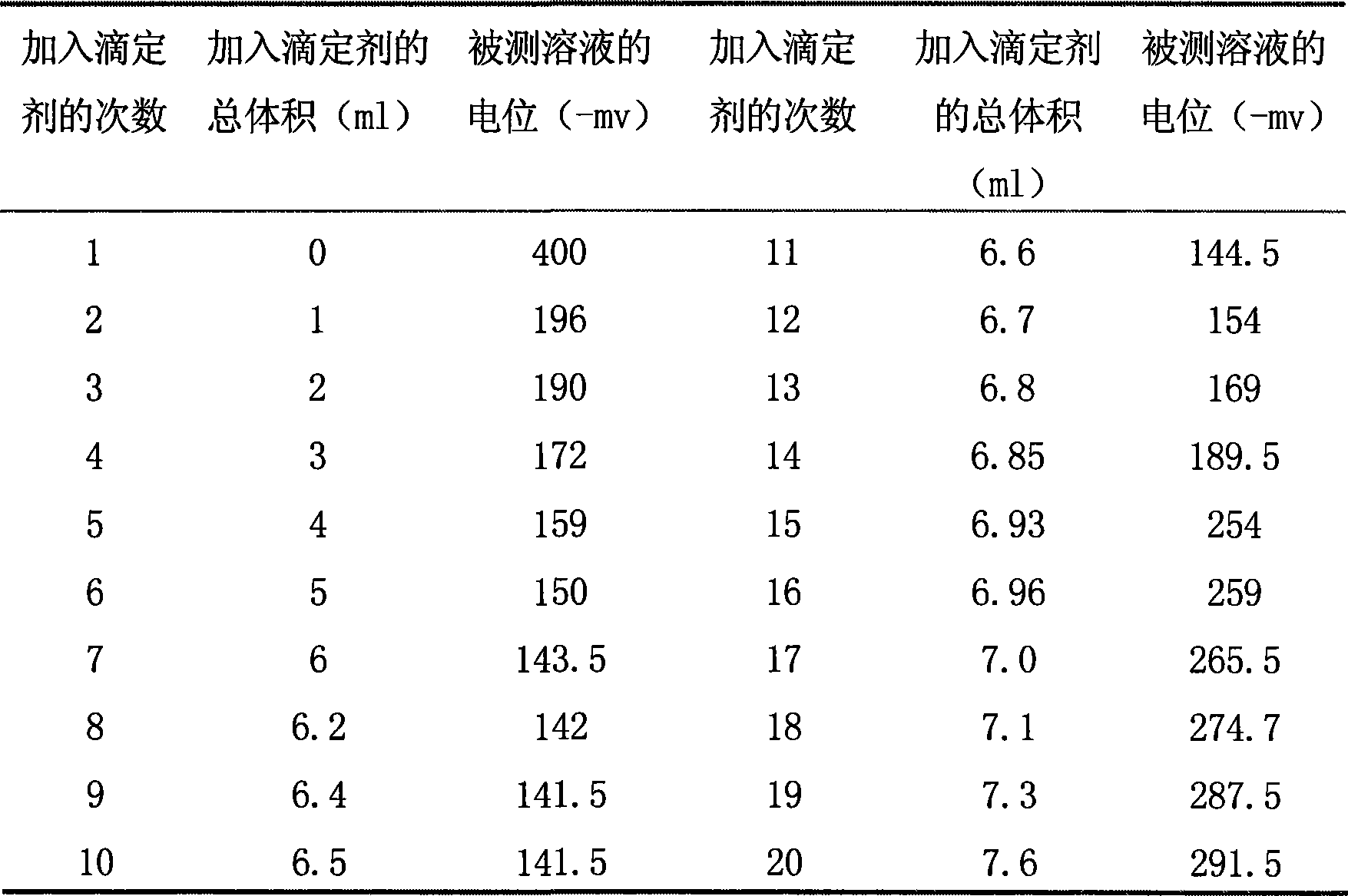
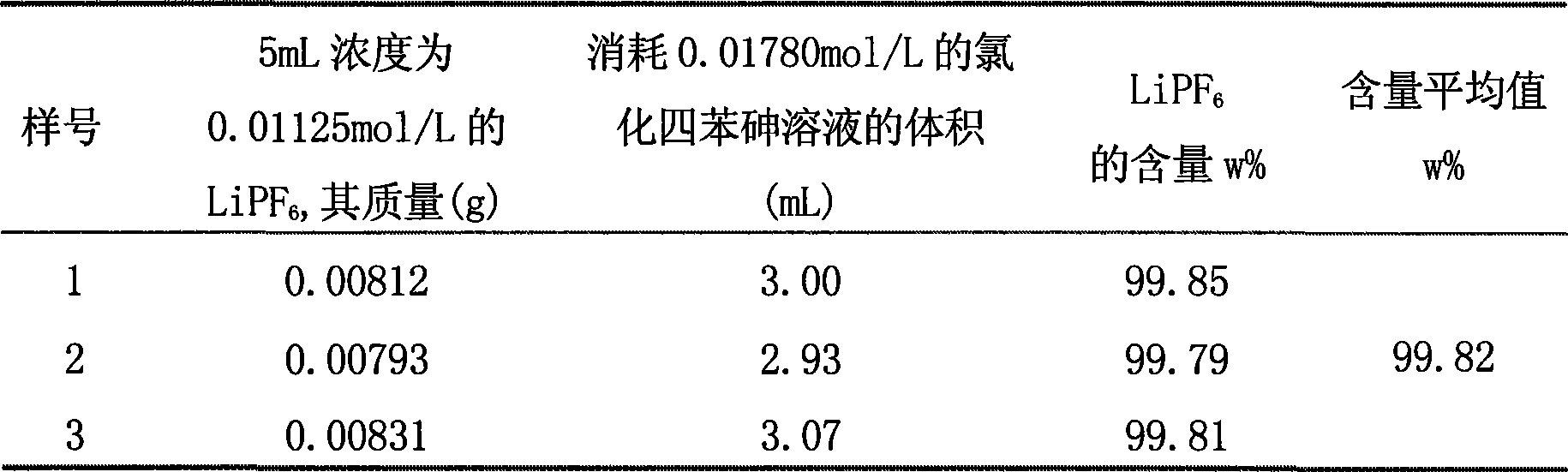
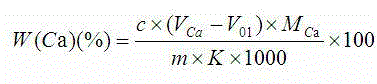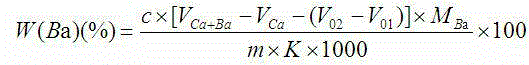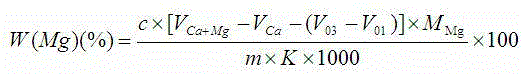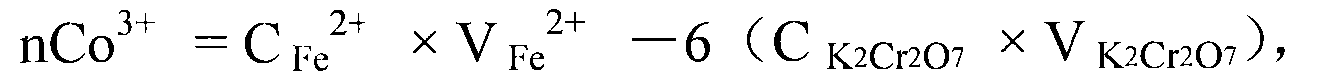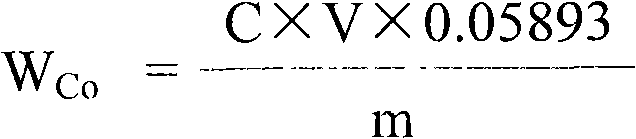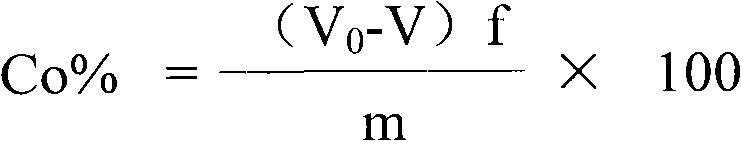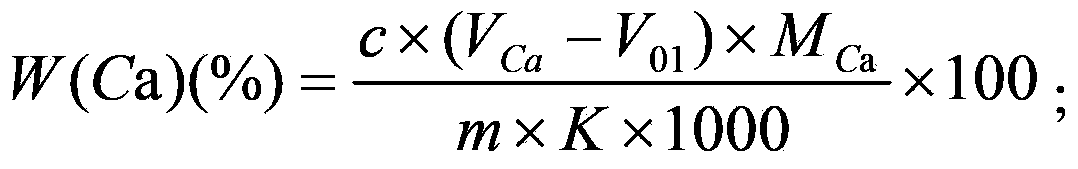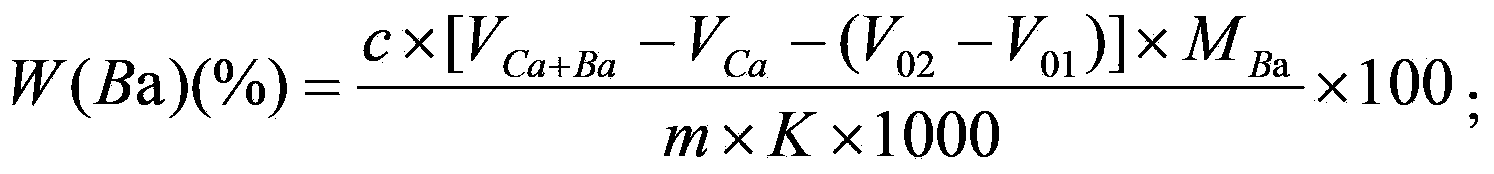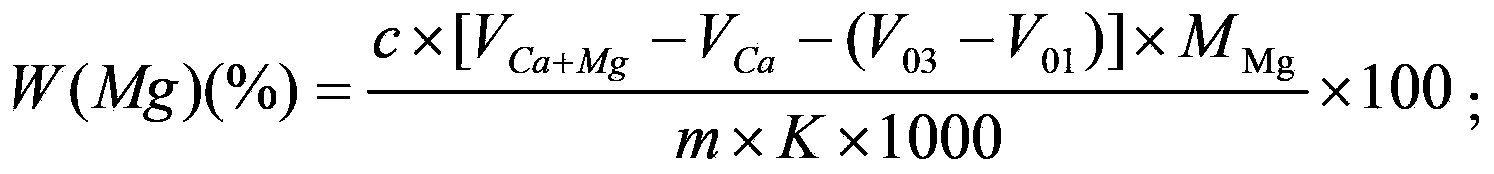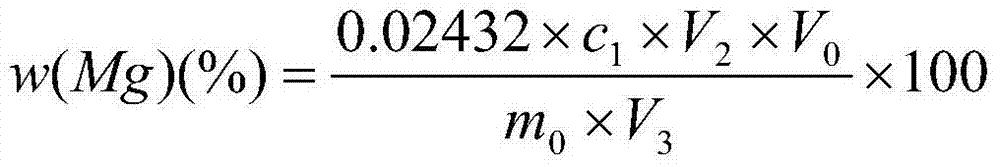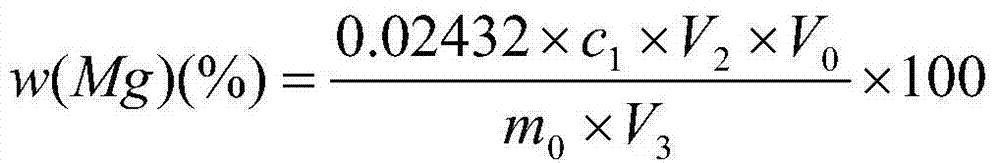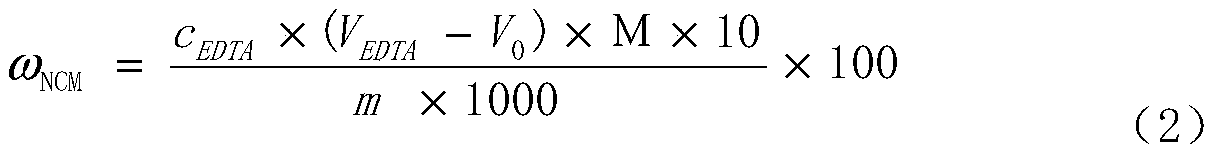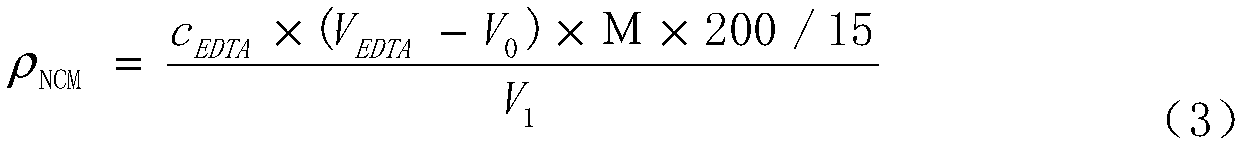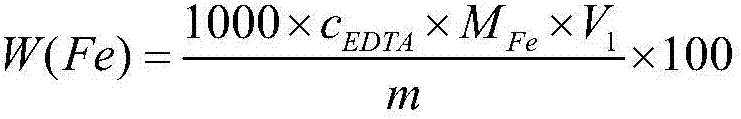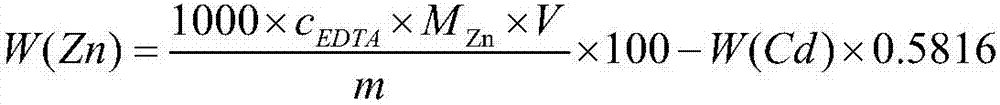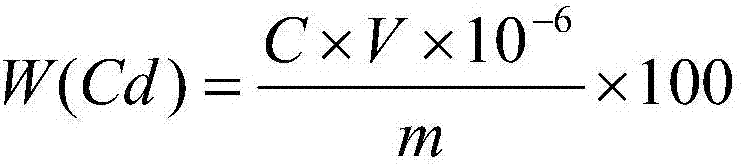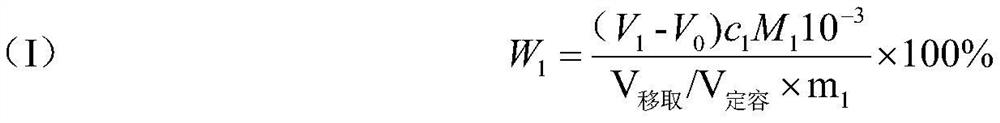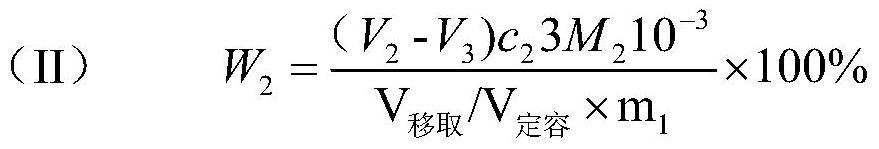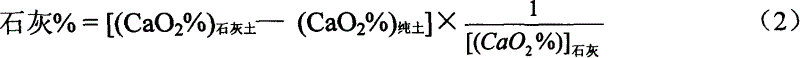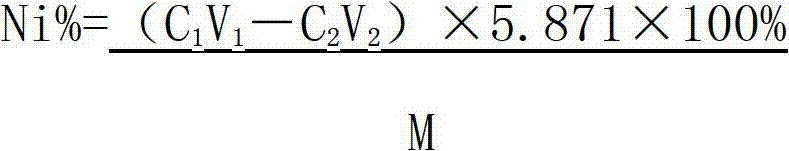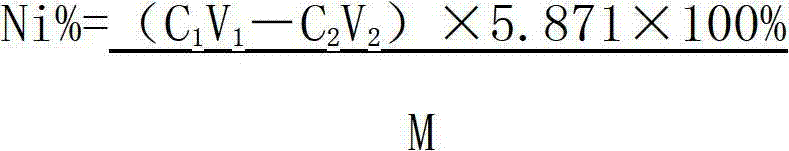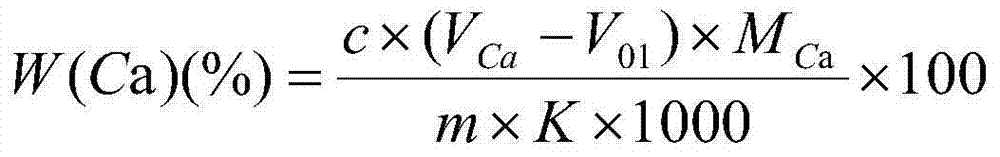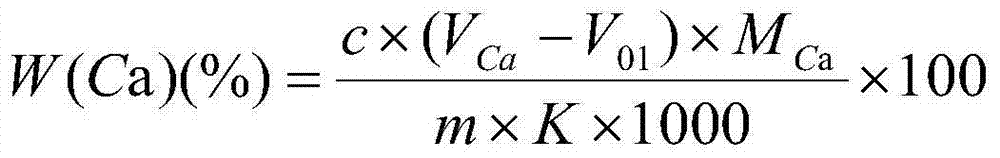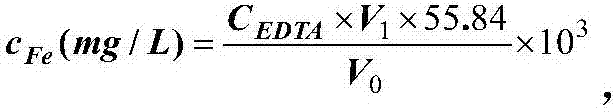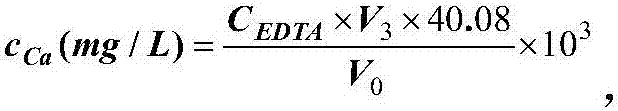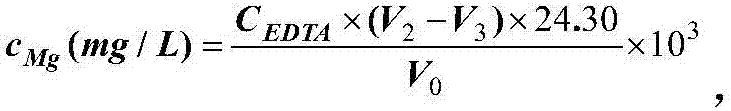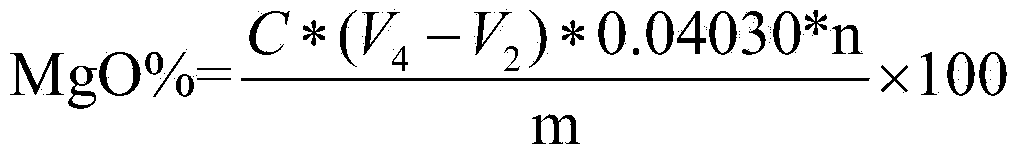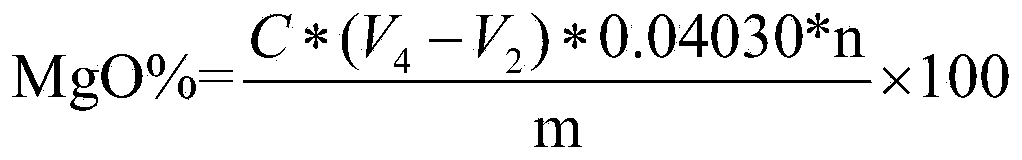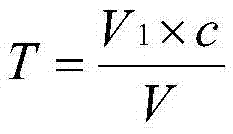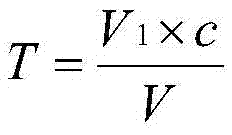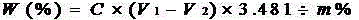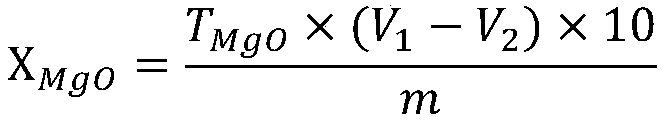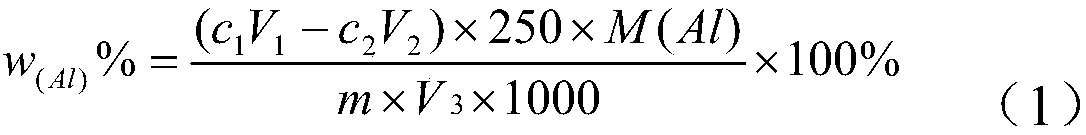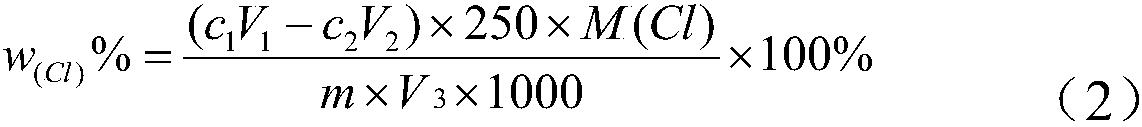Patents
Literature
66 results about "Complexometric titration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Complexometric titration (sometimes chelatometry) is a form of volumetric analysis in which the formation of a colored complex is used to indicate the end point of a titration. Complexometric titrations are particularly useful for the determination of a mixture of different metal ions in solution. An indicator capable of producing an unambiguous color change is usually used to detect the end-point of the titration .Complexometric titration are those reactions where a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators or electrometrically.
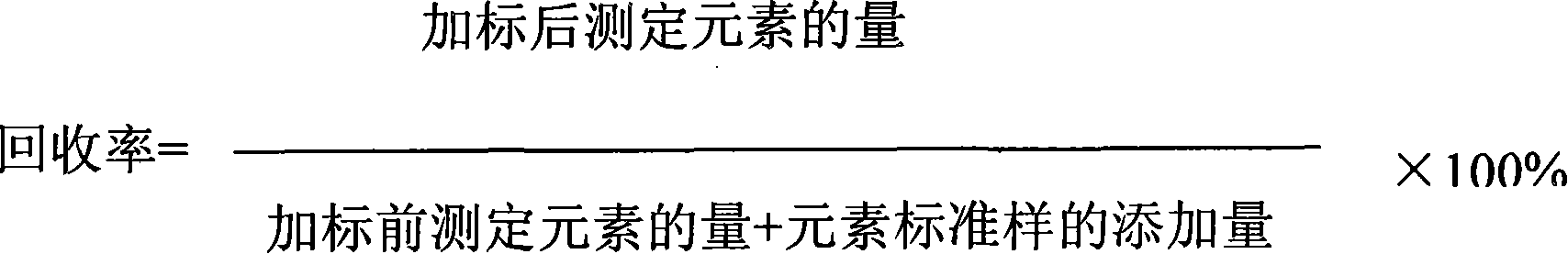
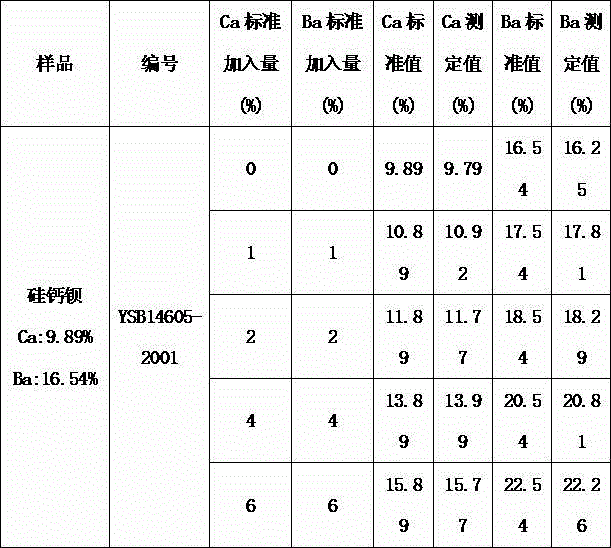
Indicator for simultaneous measurement of calcium, barium and magnesium in silicon-calcium-barium-magnesium by adopting EDTA titration method
InactiveCN104483311AColor SensitiveReduce mistakesMaterial analysis by observing effect on chemical indicatorAlizarinPhysical chemistry
Owner:INNER MONGOLIA BAOTOU STEEL UNION
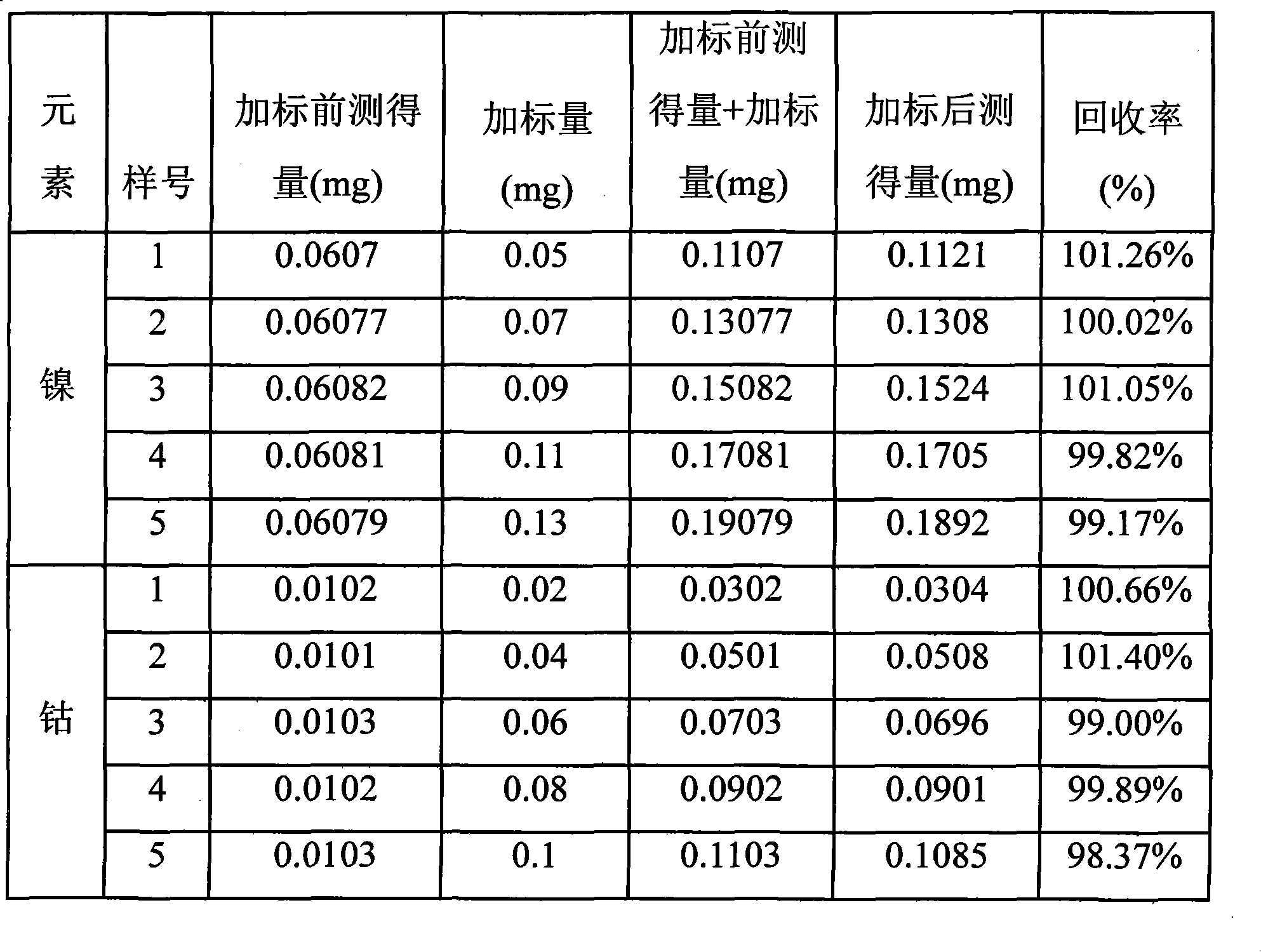
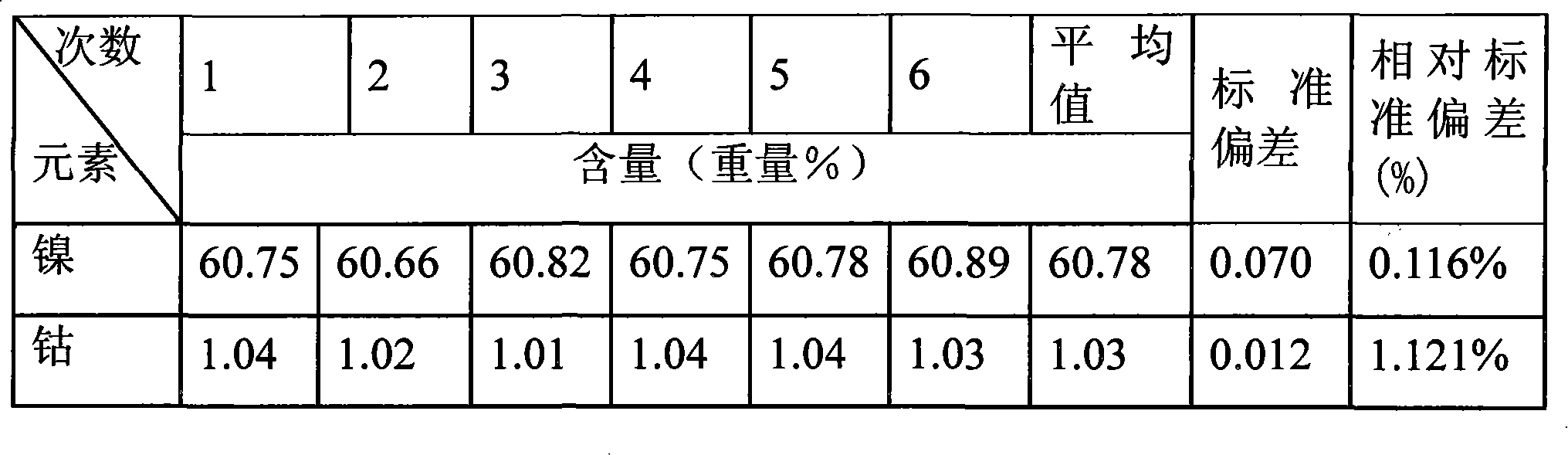
Method for testing nickel and cobalt contents in nickel compound by titration
InactiveCN101609049ALower requirementImprove accuracyMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationCobalt metalNickel compounds
The invention relates to a method for testing nickel and cobalt contents in a nickel compound by a titration, which comprises the following steps of: 1) dissolving the nickel compound to obtain nickel solution; 2) taking the partial nickel solution obtained in step 1), and carrying out complexometric titration on the nickel solution to test the nickel and cobalt metal total content; 3) taking the partial nickel solution obtained in step 1), adding ammonium persulfate, and testing the nickel metal content by using the complexometric titration again; and 4) subtracting the nickel metal content in step 3) from the nickel and cobalt metal total content tested in step 2), and obtaining the cobalt metal content. The nickel and cobalt contents in samples tested by the testing method have the advantages of small relative error and high accuracy. The testing method has the advantages of good repeatability and lower requirements of test conditions at the same time, and is suitable for work and production.
Owner:BYD CO LTD
Method for joint measurement of calcium-barium content of silicon-barium alloy by using compleximetry
InactiveCN104458731ASave operating timeReduce laborMaterial analysis by observing effect on chemical indicatorAlloyCalcium EDTA
The invention relates to a method for joint measurement of calcium-barium content of a silicon-barium alloy by using compleximetry. The method is characterized by comprising the following steps: dissolving a sample by using nitric acid and hydrofluoric acid; adding perchloric acid until smoke is generated; steaming until the sample is dried; dissolving salts by using hydrochloric acid, fixing volume by using water, then taking two samples, and adding a masking agent into the two samples respectively to eliminate interference elements; then mixing calcein with an indicator; performing titration by using EDTA (ethylene diamine tetraacetic acid) as a standard solution to measure the calcium-barium content and the calcium content respectively; and performing subtractive calculation to obtain the barium content. The method has the following advantages: by virtue of joint measurement, simultaneous measurements by one time of sample dissolving are realized, and successive measurements by two times of sample dissolving are not needed, so that the operation time is greatly shortened, and the operation is quick and accurate; by one time of sample dissolving, the labor capacity of workers is reduced, the added types and added amount of chemicals are greatly reduced, the cost is saved, the use frequency of a electric hot plate is reduced, and the energy consumption is reduced.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Measuring method of divalent cobalt content in lithium cobalt oxide
InactiveCN101685067AEasy to judgeImprove electrochemical performanceMaterial analysis by observing effect on chemical indicatorAcetic acidAmmonium ferrous sulfate
The invention belongs to a measuring method of divalent cobalt content in lithium cobalt oxide in the metallic ion quantitative detection field; the measuring method is characterized in that: total cobalt content and trivalent cobalt ion content in the lithium cobalt oxide are respectively measured, and then the total cobalt content subtracts the trivalent cobalt ion content for obtaining the divalent cobalt content in lithium cobalt oxide; wherein, the measuring method of the total cobalt content in the lithium cobalt oxide adopts ethylenediamine tetraacetic acid (EDTA) chelatometrie volumetric method, an iodometry method or a potassium ferricyanide oxidimetry method, and the measuring method of the trivalent cobalt ion content in the lithium cobalt oxide adopts an ammonium ferrous sulfate oxidimetry method. The measuring method in the invention makes up the disadvantage that the prior art has no measuring method of divalent cobalt content in lithium cobalt oxide and provides a measuring method of divalent cobalt content in lithium cobalt oxide in the metallic, wherein the method has simple operation, easy judgment of a finishing point and accurate measuring result, thereby providing powerful reference for judging the purity of the lithium cobalt oxide products and ensuring the lithium cobalt oxide products to have good electro-chemical performance.
Owner:SHENZHEN BAK BATTERY CO LTD
Complexometry joint measurement method for calcium, barium and magnesium contents in silico-calcium barium magnesium alloy
InactiveCN103512879AReduce analysis costsSave human effortMaterial analysis by observing effect on chemical indicatorEthylene diamineCalcium EDTA
The invention discloses a complexometry joint measurement method for calcium, barium and magnesium contents in a silico-calcium barium magnesium alloy. The method comprises the steps of preparing a sample solution of the alloy and a blank solution as a contrast, preparing a titration solution and a blank titration solution respectively with the sample solution and the blank solution, titrating the titration solution and the blank titration solution respectively with an EDTA (Ethylene Diamine Tetraacetic Acid) standard solution, and calculating the calcium, barium and magnesium contents in the alloy respectively according to the consumption of the EDTA standard solution, wherein the titration solution comprises a calcium blank titration solution, a calcium and barium titration solution and a calcium and magnesium titration solution, and the blank titration solution comprises a calcium blank titration solution, a calcium and barium blank titration solution and a calcium and magnesium blank titration solution. With the adoption of the method, the calcium, barium and magnesium contents can be simultaneously measured in the same solution only by once sample dissolving, and the method is easy and simple to operate.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Deep desilication method for adding composite desiliconization agent to middle and high concentration sodium aluminosilicate solution
InactiveCN101423237ASimple production processHigh silicon content indexAluminates/aluminium-oxide/aluminium-hydroxide purificationAlkali-metal aluminates/aluminium-oxide/aluminium-hydroxide preparationHigh concentrationSodium aluminosilicate
A composite desiliconization agent is added in sodium aluminosilicate solution with medium and high concentration for deep desiliconization, the invention relates to a chemical process method for carrying out the desiliconization of the sodium aluminosilicate solution with medium and high concentration by adopting the composite desiliconization agent under atmospheric pressure, the silicon content index of the solution after the desiliconization can be more than 7000, and the desiliconization method comprises the following steps: composition containing the sodium aluminosilicate solution is prepared, 80-90ml of the solution is taken and put in a reaction kettle, a calcium oxide and calcium sulfate composite desiliconization agent is added, the reaction kettle is put in a heat collection type constant temperature heating magnetic stirrer, supernatant liquor is taken after the solution is cooled, the concentration of Al2O3 is measured by using the complexometric titration, and the content of SiO2 is measured by using a spectrophotometer. The chemical process method is applicable to the secondary desiliconization of the sodium aluminosilicate solution with medium and high concentration. The economic benefits of using the sodium aluminosilicate solution with medium and high concentration are much higher than those of using the sodium aluminosilicate solution with low concentration under the situation of the same energy consumption, thereby being beneficial to reducing the energy consumption and promoting the sustainable development of the production of aluminum oxide.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Biological method for recycling rare earth from low-concentration heavy yttrium rare earth wastewater
InactiveCN107312933AEliminate hazardsReduce operating costsWater contaminantsProcess efficiency improvementRare earth ionsDesorption
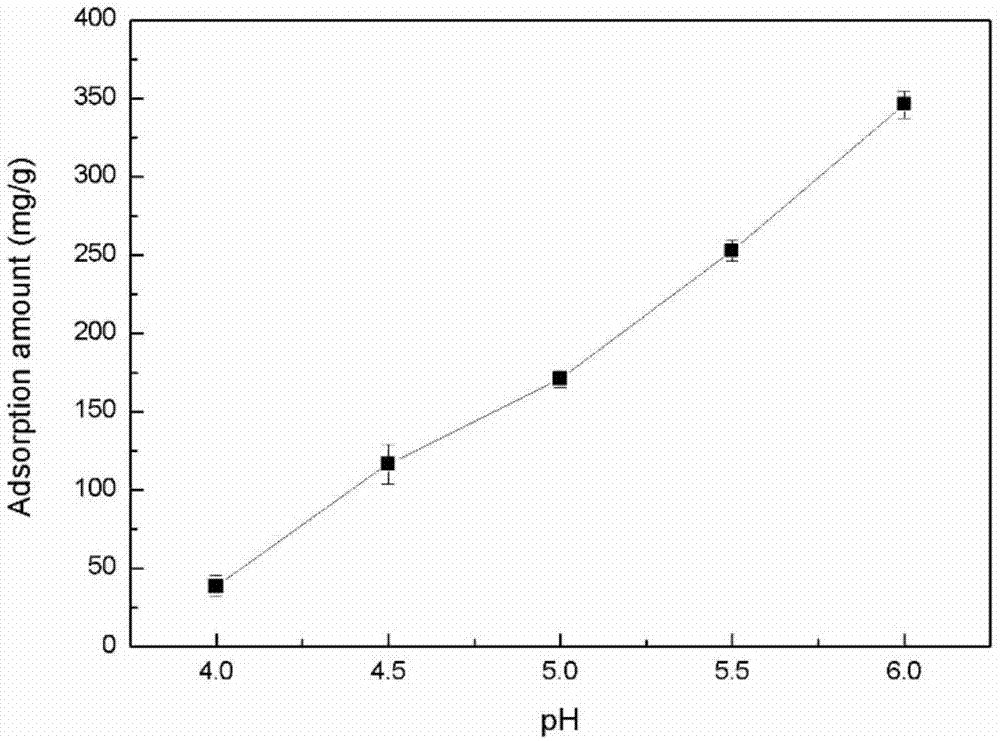
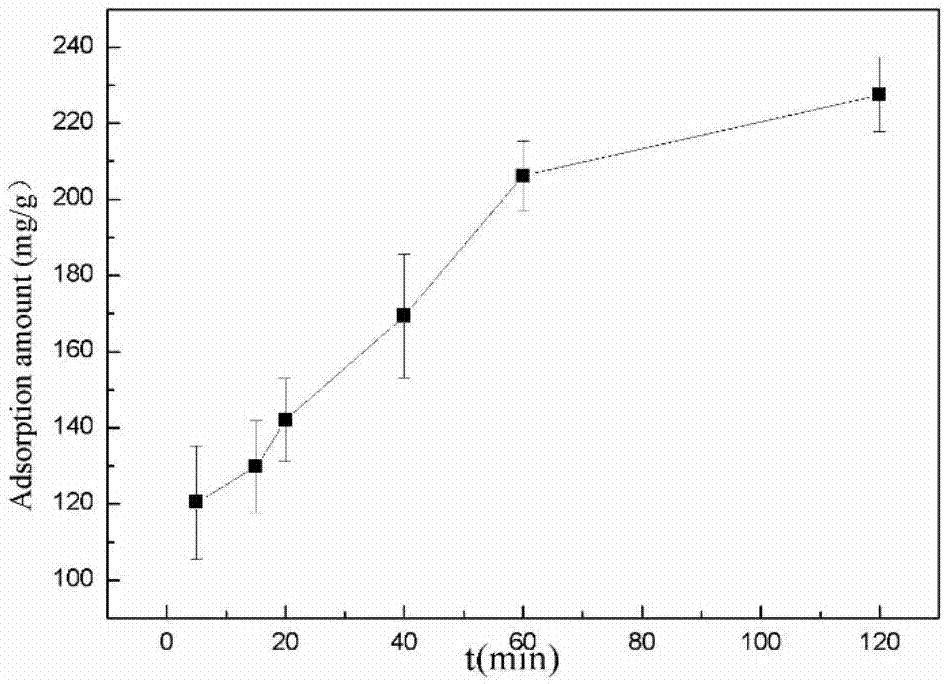
The invention provides a biological method for recycling rare earth from low-concentration heavy yttrium rare earth wastewater, and belongs to the biotechnology field. The method includes the steps that a liquid beef extract-peptone medium is used for cultivating Serratia marcescens, and thalli are harvested; then a rare earth solution with the initial concentration being 10-200mg / L is added with the Serratia marcescens, constant temperature vibration is conducted under the conditions that the pH is 4-5.5, the temperature is 20-40 DEG C and the rotating speed is 50-400r / min, then an adsorption solution is subjected to centrifuging under the condition of 8000*g at the temperature of 4 DEG C, and rare earth ion adsorption thalli are harvested; an EDTA complexometric titration method is used for measuring the concentration of supernate rare earth ions, and the rare earth ion adsorbing capacity is calculated; and finally, the thalli with the given rare earth ion adsorbing capacity are added into a desorption reagent, constant temperature vibration is conducted for desorption, and a desorption solution is subjected to centrifuging under the condition of 8000*g at the temperature of 4 DEG C so that thalli can be harvested; and the EDTA complexometric titration method is used for measuring the concentration of the supernate rare earth ions, and the rare earth ion desorption rate is calculated. By the adoption of the method, damage to the environment by the rare earth ions can be removed, rare earth resources are recycled from the low-concentration wastewater, the process is simple and practicable, operation cost is low and the method is environmentally friendly.
Owner:JIANGXI UNIV OF SCI & TECH
Method for measuring metallic magnesium content of briquette nodulizing agent by using complexometry
ActiveCN104122366ADissolve fastReduce solubilityMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationVolumetric flaskAccuracy and precision
The invention discloses a method for measuring the metallic magnesium content of a briquette nodulizing agent by using complexometry. The method comprises the steps of dissolving metallic magnesium in a test sample in dilute acetic acid, removing other Insoluble matters through filtering and washing, and fixing the volume of the filtrate in a volumetric flask; and weighing a certain volume of filtrate, regulating the pH value to 6-7 with ammonia water, separating interference elements with a copper reagent, complexing calcium with EGTA (ethylene glycol tetraacetic acid) when the pH is 10, and performing complexometry on magnesium by using EDTA (ethylene diamine tetraacetic acid), wherein eriochrome black T is an indicator. The method can detect that the metallic magnesium content in a briquette nodulizing agent sample is 5-15%, which nearly covers the metallic magnesium distribution gradients of all nodulizing agents in the production process, and can be applied to quantitative detection of the metallic magnesium in the laboratory briquette nodulizing agent for a long term. The method has the advantages of simplicity, fastness, high accuracy and precision and the like.
Owner:INST OF RES OF IRON & STEEL JIANGSU PROVINCE
Method for determining total amount of nickel, cobalt and manganese in NCM material through complexometric titration method
InactiveCN108918752AThe end color changes obviouslyImprove stabilityChemical analysis using titrationSpecific testManganese
The invention discloses a method for determining the total amount of nickel, cobalt and manganese in a NCM material through a complexometric titration method. In the prior art, the rapid test of the total amount of nickel, cobalt and manganese is performed by using the EDTA complexometric titration method, and a lot of differences exist in the specific test operations. According to the present invention, the auxiliary reagent hydroxylamine hydrochloride is additionally used in the complexometric titration method, wherein the color change at the ending point is significant and the test result is stable and accurate by adding the reagent; during the titration test, the buffer solution and the murexide indicator are added at 1-2 ml position before the ending point, such that the test result is stable and accurate; with the method, the total amount of nickel, cobalt and manganese in the NCM material can be quickly, accurately and stably determined, wherein the test result is close to the real value by verifying the standard sample; and the method has characteristics of low requirements on operators, easy grasping, good reproducibility of test result and significantly-shortened test time, and can completely meet the requirements of large-scale and continuous production.
Owner:HUAYOU NEW ENERGY TECH (QUZHOU) CO LTD +1
Method for continuously determining zinc content and iron content of zinc concentrate
InactiveCN107132219AGuaranteed accuracyImplement batch operationsMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationInterference factorZinc
The invention belongs to the technical field of mineral content analysis, and discloses a method for continuously determining the zinc content and the iron content of zinc concentrate in order to solve the tediousness of two sample dissolving processes needed by individual determination. The method comprises the following steps: decomposing a sample by using hydrochloric acid, nitric acid, ammonium fluoride and sulfuric acid, adding ammonia water, separating the obtained precipitate, processing the obtained filtrate, determining zinc through using a Na2EDTA complexing titration technology, processing the obtained precipitate, and determining iron by using the Na2EDTA complexing titration technology. The method is very convenient to operate. Compared with existing other continuous titration methods, the method using the same standard EDTA solution in the invention has the advantages of simplification of the experiment program, reduction of the experiment cost, increase of the test speed of the sample, effective increase of the working efficiency, few experiment interference factors, and increase of the accuracy.
Owner:CHANGCHUN GOLD RES INST
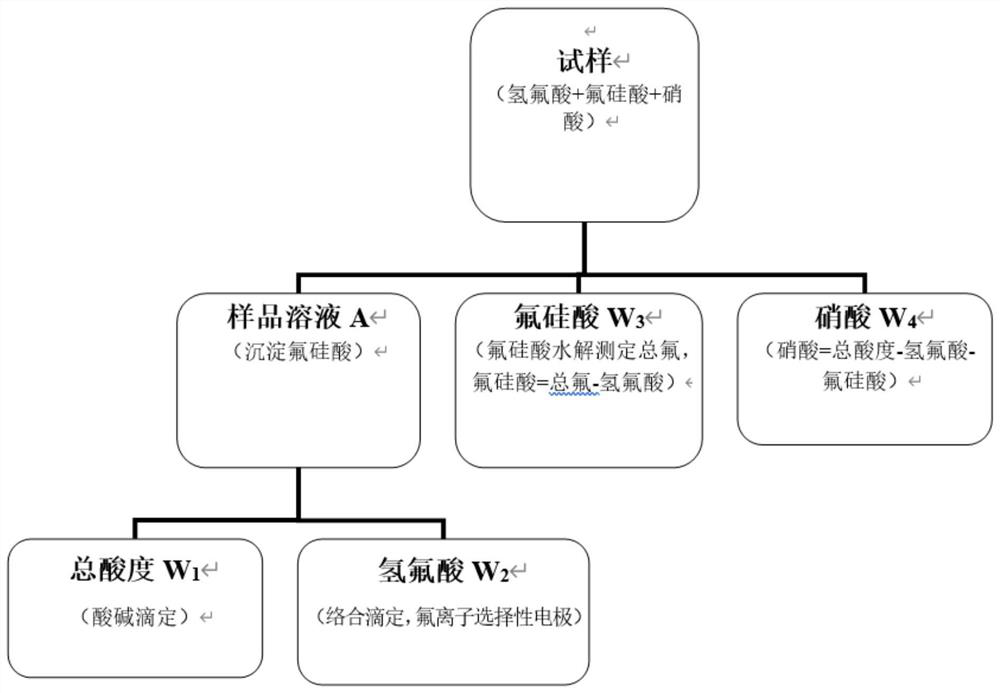
Method for determining contents of fluosilicic acid, hydrofluoric acid and nitric acid in etching acid
InactiveCN111855650AEasy to operateReliable resultsMaterial analysis by observing effect on chemical indicatorMaterial electrochemical variablesHexafluorosilicic acidLanthanum
The invention discloses a method for determining the contents of hydrofluoric acid, fluosilicic acid and nitric acid in etching acid through acid-base titration and complexometric titration. The method comprises the following steps: (1) adding potassium salt into a sample to precipitate fluosilicic acid, titrating supernatant by using a sodium hydroxide standard titration solution by taking phenolphthalein as an indicator, and calculating the total acidity of the sample solution; (2) adding potassium salt into the sample to precipitate fluosilicic acid, titrating fluorine ions in the solutionby using a lanthanum nitrate standard titration solution by taking a fluorine ion selective electrode as an indicating electrode, determining a reaction endpoint by using a secondary micro-commerce method, and calculating the content of hydrofluoric acid; (3) heating and hydrolyzing fluosilicic acid, titrating total fluorine in the solution by using a lanthanum nitrate standard titration solutionby taking a fluorine ion selective electrode as an indicating electrode, determining a reaction endpoint by using a secondary micro-commerce method, and calculating the content of fluosilicic acid byusing a subtraction method; and (4) obtaining the nitric acid content by subtracting the sum of the hydrofluoric acid content and the fluosilicic acid content from the total acidity.
Owner:QINGDAO UNIV OF SCI & TECH
Method for determining lime dosage in long-life limy stabilized soil
InactiveCN1609613ASolving Metering ProblemsAccurate measurementChemical analysis using titrationPreparing sample for investigationEthylenediamineVolume concentration
The determining process of lime content in long term lime stabilized soil includes the following steps: weighing lime soil sample, adding hydrochloric acid solution in the concentration of 50 vol% in 50 ml to dissolve the lime soil via stirring, transferring the supernatant into conical flask, complex titrating with standard 0.05 mol / L concentration EDTA solution and determining the volume of consumed standard 0.05 mol / L concentration EDTA solution Vlime soil; determining the Vpure soil and Vlime in similar process; and calculating the percentage content of lime in the lime soil based on corresponding formula. The method is simple, accurate and widely applicable.
Owner:CHANGAN UNIV
Method determining content of nickel in nickel-titanium shape memory alloy
ActiveCN102928422AAccurate control of acidityAcidity controlMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationAlloyMethyl orange
The invention relates to a method for determining the content of nickel in a nickel-titanium shape memory alloy. The method comprises the following steps of: dissolving a sample with ammonium fluoride and nitric acid, and fluorating the sample; adding excessive EDTA (Ethylene Diamine Tetraacetic Acid) to make the EDTA completely complexed with nickel; regulating the acidity through an indicator; adding a buffer solution to control the acidity; titrating excessive EDTA by using a zinc scale; and determining the content of nickel by calculating. According to the method, the influence of titanium on the nickel during analysis is eliminated by masking the titanium, the end point mutation is obvious when the solution is titrated by regulating the acidity, and the more accurate content of nickel elements can be acquired by analyzing iron elements. The method for determining the content of the nickel in the nickel-titanium alloy has the advantages of simple process, obvious titration end point mutation, high recovery rate and high precision; the influence of Fe3<+> on the determination of the nickel can be eliminated; and the defects that methyl orange is used as an indicator to regulate the acidity, and xylenol orange is used as an indicator of complexometric titration end points in the conventional method to cause that the acidity control deviation is larger, and the titration end points are not obvious are overcome.
Owner:AECC AVIATION POWER CO LTD
Method for measuring magnesium content in fluxing agent II
ActiveCN103091453ALow costAnalysis using chemical indicatorsChemical analysis using titrationPotassiumManganese
The invention provides a method for measuring magnesium content in a fluxing agent II. The method comprise the following steps of: putting a fluxing agent II sample to water, heating up and filtering the mixture to obtain filter residue; mixing and dissolving the filter residue with hydrochloric acid and nitric acid to obtain a solution; adjusting the pH value of the solution, adding a copper reagent to the solution, and filtering the solution to obtain filtrate; carrying out calcium-magnesium titration and calcium titration, and obtaining the content of the magnesium oxide through conversion of volume of a sodium ethylene diamine tetracetate standard titration solution which is consumed by the twice titration. Compared with an atomic absorption spectrometry or an ICP (Inductively Coupled Plasma) spectrometry in the prior art, soluble impurities such as un-reacted magnesium chloride, un-reacted potassium chloride and the like are firstly dissolved and removed by water; the magnesium oxide is dissolved by the mixture of hydrochloric acid and nitric acid; the interfering ions such as iron, manganese, aluminum, nickel, copper and the like in the solution are precipitated and separated by the copper reagent; and the content of the magnesium oxide is obtained through the conversion utilizing EDTA (Ethylene Diamine Tetraacetic Acid) complexometric titration. Therefore, the content of the magnesium oxide can be measured by only using simple glass wares and conventional chemical reagents without much investment, and the cost is low.
Owner:SOUTHWEST ALUMINUM GRP
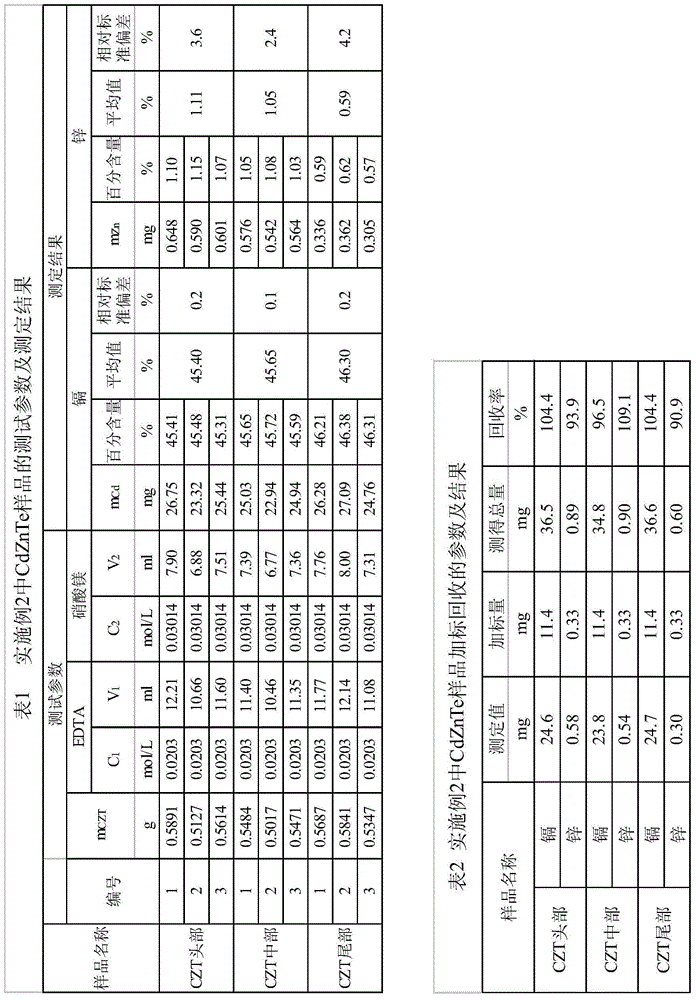
Method for testing constant cadmium and zinc in cadmium zinc telluride crystal
ActiveCN104914093ASolve the problem that titration cannot be carried outSuppress interferenceMaterial analysis by observing effect on chemical indicatorTe elementCadmium Cation
The invention provides a method for testing constant cadmium and zinc in cadmium zinc telluride crystal. The method provided by the invention comprises the following steps: taking a solution obtained by dissolving a proper amount of a sample solution of the cadmium zinc telluride crystal to be tested or a proper amount of a solid sample of the cadmium zinc telluride crystal to be tested, adjusting pH value of the solution to neutral by adding ammoniacal liquor (1+1) to and adjusting pH value of the solution to 10 by adding a proper amount of an ammonia-ammonium chloride buffer solution and remaining stable; adding a proper amount of a surfactant into the solution, adding a proper amount of an eriochrome black T indicator, and carrying out complexometric titration by the use of a complexing agent EDTA to turn the solution from purple to pure blue so as to obtain total amount nCd+Zn of cadmium and zinc, wherein molar concentration of the complexing agent EDTA is C1 and the use amount is V1; adding a proper amount of DDTC sodium salt to fully dissolve, selectively precipitate cadmium by DDTC sodium salt and releasing the same amount of EDTA, carrying out titration on EDTA by the use of a magnesium nitrate solution until the solution turns light red so as to obtain cadmium with the amount of nCd and mass of mCd, wherein molar concentration of the magnesium nitrate solution is C2 and use amount is V2; and obtaining zinc with amount of nZn and mass of mZn according to a difference method.
Owner:清远先导材料有限公司
Quality method complexometric titration nickel-cobalt-manganese content detection method
InactiveCN111735903AAccurate detectionMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationPhysical chemistryManganese
The invention belongs to the field of detection, and particularly relates to a mass method complexometric titration nickel-cobalt-manganese content detection method. The method comprises the followingsteps: 1, digesting and diluting a battery material containing nickel, cobalt and manganese to a set mass constant volume, performing titrating by using an EDTA standard solution, and calculating thetotal content of nickel, cobalt and manganese according to the amount of the consumed EDTA standard titration solution; 2, dissolving the battery material containing nickel, cobalt and manganese by adopting acid, and detecting the proportion of nickel, cobalt and manganese through an inductively coupled plasma emission spectrum ICP; 3, calculating the contents of nickel, cobalt and manganese according to the result of the step 1 and the result of the step 2. The method has the advantages that multiple tiny influence factors such as actual operation constant volume, pipetting volume and measuring tool specification errors, environment temperature and volume relation factors, measuring tool solution wall sticking residues and influences, tiny variation factors of volume caused by degradation and co-dissolution of digestion acid concentration and the like are eliminated; meanwhile, the accurate total amount and weight of the nickel-cobalt-manganese hydroxide are accurately detected.
Owner:YINGDE KEHENG NEW ENERGY TECH CO LTD
Calcium content determination method for low-calcium aluminum ferromanganese
ActiveCN104515828AAccurate measurementDetermination is accurate and stableChemical analysis using titrationSal ammoniacCalcium content
The invention discloses a calcium content determination method for low-calcium aluminum ferromanganese. The calcium content determination method comprises the following steps: utilizing hydrochloric acid-hydrogen peroxide to dissolve a sample; utilizing ammonia water to separate out aluminum, iron and other elements; using ammonium persulfate to oxidize and remove manganese to form colloidal precipitate; after filtering and separating out the precipitate, repeating the manganese removing process; adding hydrochloric acid to a manganese-removing solution; boiling the solution to remove superfluous ammonium persulfate; using ammonia water to regulate the pH value to be 7; splitting a part of solution; adding a masking agent to eliminate other ion interference; carrying out complexometric titration to determine the calcium element content. With application of the determination method disclosed by the invention, the calcium content in low-calcium aluminum ferromanganese can be accurately, stably, simply and efficiently determined, and the determination for the calcium content in the low-calcium aluminum ferromanganese can be faster and more convenient, so that the determination cost can be reduced.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Method for testing content of titanium dioxide by complexometric titration-back titration method
InactiveCN103091320AAvoid easy changeReduce usageMaterial analysis by observing effect on chemical indicatorBack titrationBuffer solution
The invention provides a method for testing content of titanium dioxide by a complexometric titration-back titration method. The method comprises the following steps of: (1) treating a sample; (2) optimizing an operation process, and reasonably adjusting the concentration of solution pH alkali; and (3) adding a buffer solution; and then calculating out the content of the titanium dioxide in the sample. By adopting the method provided by the invention, the acidity of the solution at the end point of titration can be controlled well, an indicator is ensured to change the color obviously, the precision of sample test is improved, and the relative deviation of the sample test result is less than 0.3%.
Owner:JIANGSU ALAND NOURISHMENT
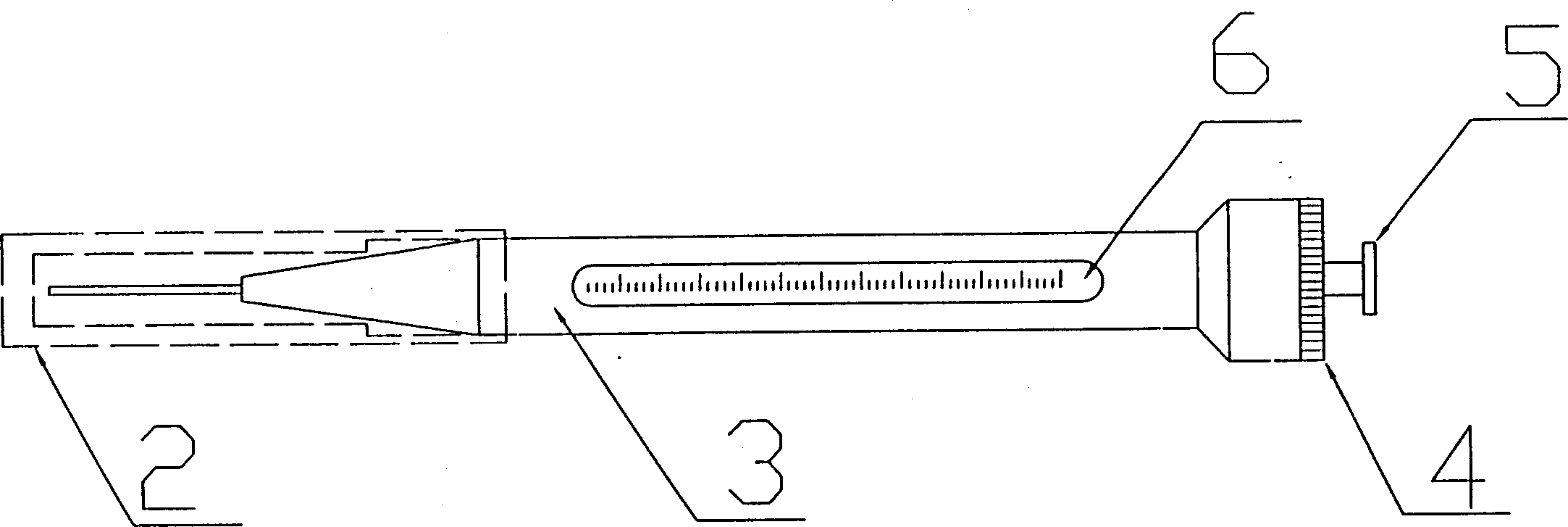
Hand-held micro titrator
InactiveCN1891340AEasy to useAccurate drop volumeChemical analysis using titrationBurettes/pipettesHand heldEngineering
This invention discloses a handheld minim titrator including a sample pin protection jacket, a titrator wrap, a core locking plug of the titrator, a sample cavity of the sample pin and the titrator, a titrator pull rod and a core characterizing that said titrator pull rod is set in the sample cavity of the sample pin and the sample titrator to make up of a core, which is set in the wrap to be locked by the plug of the core and the protection jacket of the sample pin coveres one end of the pin to form a handheld minim titrator, which can replace acid-alkali buret in the lab and is suitable for oxidation-reduction titration method.
Owner:CHANGCHUN JILIN UNIV LITTLE SWAN INSTR
Method for quick quantitative analysis of metal cations in oil field water
InactiveCN107064129AThe assay does not interfereReduce stepsMaterial analysis by observing effect on chemical indicatorIron removalWater quality
The invention discloses a method for quick quantitative analysis of metal cations in oil field water. By complexometric titration, synchronous titration of calcium, magnesium, strontium, barium and iron in an oil field water sample is realized through solution acidity control and precipitation masking without special pretreatment. Due to reduction of steps of iron removal, heating, aging, filtering and diluting, operation steps are greatly reduced, operation time is greatly shortened, measurement result accuracy is improved, and operation efficiency is improved as well. The method is hopefully popularized and applied in oil field water quality analysis.
Owner:XI'AN PETROLEUM UNIVERSITY
Method for testing magnesium oxide in talcum powder
InactiveCN103529166AAccurate measurementEasy to operateChemical analysis using titrationPhenanthrolineMuffle furnace
The invention relates to a method for testing magnesium oxide in talcum powder. The method specifically comprises the following steps: putting a certain amount of the talcum powder into a nickel crucible, adding a corresponding amount of solid NaOH, smelting in a muffle furnace of 700-750 DEG C, acidizing through hydrochloric acid and performing complexometric titration through an ethylene diamine tetraacetic acid (EDTA) standard solution. In the titration process, interference of Fe ions, Al ions and Ni ions as the nickel crucible is used can be prevented by taking a triethanolamine solution together with phenanthroline as a masking agent. The method is simple and convenient to operate, accurate in testing result and both economical and time-saving.
Owner:HUBEI FORBON TECH
Chemical analysis determination method of zinc content of molecular sieve
InactiveCN105445414AEffective maskingEasy to operateChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorMolecular sieveTiter
The invention provides a chemical analysis determination method of zinc content of molecular sieve. The chemical analysis determination method comprises following steps: titer T of EDTA standard solution on zinc is obtained via calibration; a sample is dissolved in a strong base, and the pH value of an obtained mixture is adjusted to be lower than 7; a masking agent is added; and EDTA complexometric titration is adopted to determining the content of zinc in molecular sieve. In the chemical analysis determination method, the strong base is adopted to dissolve the sample; and ammonium fluoride and tartaric acid are taken as the masking agent; so that influences of interfering ions on determination results are avoided; the operation steps of the chemical analysis determination method are simple; and accuracy is high.
Owner:天津神能科技有限公司
Method for detecting metal ion contents in zinc stearate and calcium stearate composite salt
InactiveCN103512881ASimple methodEasy to operateMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationStearateZinc stearate
The invention discloses a method for detecting metal ion contents in zinc stearate and calcium stearate composite salt. The method comprises the following steps: accurately weighing composite stearate, adopting a complexometric titration method, utilizing the different capacities of complexing metal ions of an EDTA sodium solution under different acid and alkaline conditions, recording the volume of the consumed EDTA sodium solution, and calculating the content of each metal ion. The method provided by the invention is simple and easy to operate, and the detected stearate metal contents are accurate and reliable.
Owner:NANTONG XINBANG CHEM
Method for measuring content of free boron oxide in boron nitride
InactiveCN105372386AHighlight substantive featuresSignificant progressMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationBoron nitrideBoron oxide
The invention provides a method for measuring the content of free boron oxide in boron nitride. The method specifically comprises the following steps: boron nitride is subjected to acid pickling in an acid solution, filtering and acid-base neutralization titration, a neutral mixed solution is prepared and subjected to complexometric titration and acid-base neutralization titration by adopting an alcohol complexing agent and a strong base standard solution respectively, the volume difference of the consumed strong base standard solution is obtained through a blank test, and an established unary linear quantitative detection model for free boron oxide in boron nitride is used for calculation, so that the content of free boron oxide in boron nitride is obtained. The method has simple steps and high detection result accuracy.
Owner:FUNIK ULTRAHARD MATERIAL
Method for measuring hexafluorophosphoric acid radical ion by amperometric titration
InactiveCN101464426ARapid determinationAccurate measurementMaterial electrochemical variablesAmperometric titrationLyonium ion
The invention relates to a method for analyzing and measuring hexafluorophosphoric acid ions, in particular to a method for measuring hexafluorophosphoric acid ions through the amperometric titration. The method comprises the following steps: (1), preparing a standard tetraphenylarsonium chloride solution, and calibrating the concentration of the tetraphenylarsonium chloride solution; (2), preparing a hexafluorophosphoric acid ion solution to be tested; (3), setting conditions for polarographic analysis: the initial potential ranges from minus 1.25 to minus 1.78; the sensitivity I is 8; the differentiation is 2.0; the scanning is carried out once; the step length is 5; the hexafluorophosphoric acid ions are subjected to the amperometric titration, with tetraphenylarsinium chloride taken as a titrant; and (4), reducing excess tetraphenylarsonium ions on mercury drops after (C6H5)4AsPF6 is produced, and calculating the concentration of a lithium-ion battery electrolyte sample to be tested on the basis of the concentration of lithium salt in a diluted sample and the dilution multiple. The invention provides the quick, accurate and economical method for measuring the hexafluorophosphoric acid ions. Tetraphenylarsonium chloride can remain in a stable state in a solution, tetraphenylarsonium chloride and hexafluorophosphoric acid salt form complex compound sediment, and the hexafluorophosphoric acid ions are unlikely to be oxidized or reduced by the mercury drops, so that the reducibility of the tetraphenylarsonium ions facilitates the analysis of the hexafluorophosphoric acid ions.
Owner:NORTHWEST RES INST OF MINING & METALLURGY INST
Method of detecting periclase content in cement clinker
ActiveCN109374613AThe test results are true and accurateGood dispersionMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationAlcoholMass ratio
The invention discloses a method of detecting periclase content in cement clinker and belongs to the technical field of detection of building materials. The method comprises the steps of S01, crushingcement clinker, crushing, and screening to obtain a clinker sample; S02, mixing castor oil and anhydrous ethyl alcohol in a mass ratio of (10-15):1 to obtain a shielding agent; S03, adding 1 part bymass of the clinker sample into 40-80 parts by mass of the shielding agent, and holding the temperature of 50-70 DEG C for 10-20 min; S04, adding 40-60 parts by mass of ammonium nitrate and 150-200 parts by mass of anhydrous ethyl alcohol to the obtained material of step S03, heating at 85-95% to allow refluxing for 1-4 h, performing suction filtering while the temperature is high, washing the precipitate with hot anhydrous ethyl alcohol, and combining the washed precipitate with the filtrate; S05, cooling the filtrate of the step S04 to room temperature, and detecting periclase content by means of complexometric titration difference process. The method herein is suitable for more efficiently separating solid-solution magnesia and periclase, detection results for periclase are more real and accurate, and stability of the cement clinker can be correctly estimated.
Owner:湖北建研科峰工程质量检测有限公司
Method for testing content of alkyl chloride aluminum for ethylene-propylene polymerization
InactiveCN108226372AThe method is simpleThe test result is accurateChemical analysis using titrationNitrogenChloride
The invention discloses a method for testing the content of alkyl chloride aluminum for ethylene-propylene polymerization. The method comprises the following steps: performing dehydration treatment onalkyl chloride aluminum in a vessel, performing titration to test the content of aluminum by using a complexing titration method, and further performing titration to test the content of chloride by using a Volhard method. By adopting the method, a conventional alkyl chloride aluminum content testing method is changed, the method does not need to be carried out in a nitrogen tank, and only reactions and hydration treatment are carried out in a ventilation cabinet. The method is simple, convenient and feasible. The hydration treatment steps are carried out in relatively special vessels, an alkyl chloride aluminum sample is added in a completely sealed environment in the testing process, the sample and a hydrogen chloride gas generated from reactions are not lost, and precise testing resultscan be achieved.
Owner:PETROCHINA CO LTD
Accurate determination method for lead content in white soot containing bismuth
InactiveCN109557246AEliminate distractionsAnalysis process is shortChemical analysis using titrationPreparing sample for investigationSodium acetateLead sulfate
The invention provides an accurate determination method for lead content in white soot containing bismuth, and belongs to the determination method for the lead content in the white soot. The method comprises the following steps of: dissolving a sample by using ammonium fluoride solid, nitric acid, sulfuric acid and hydrobromic acid, forming lead sulfate precipitate in the sulfuric acid medium, filtering and separating from most coexisting elements; dissolving the lead sulfate precipitate in acetic acid-sodium acetate buffer solution; using xylenol orange solution as an indicator when the pH value is adjusted from 1.40 to 1.60 with nitric acid; performing complexation titration with a Na2EDTA titration solution which does not complex with lead to remove bismuth; and adjusting the pH to 5.40to 5.60 with ammonia water and titrating with a Na2EDTA standard titration solution; and calculating the lead content according to the consumed volume of the Na2EDTA standard titration solution. Theadvantage is that: the addition of hydrobromic acid can effectively remove the interference of arsenic, selenium and antimony in the sample; the pH is adjusted, the interference of the bismuth can beeliminated by the titration with the dilute Na2EDTA standard solution at a pH of 1.40 to 1.60; the analysis process is short, the operation is simple, and the work efficiency is effectively improved.
Owner:CHANGCHUN GOLD RES INST
Measurement method for zirconium oxide in drainage sand
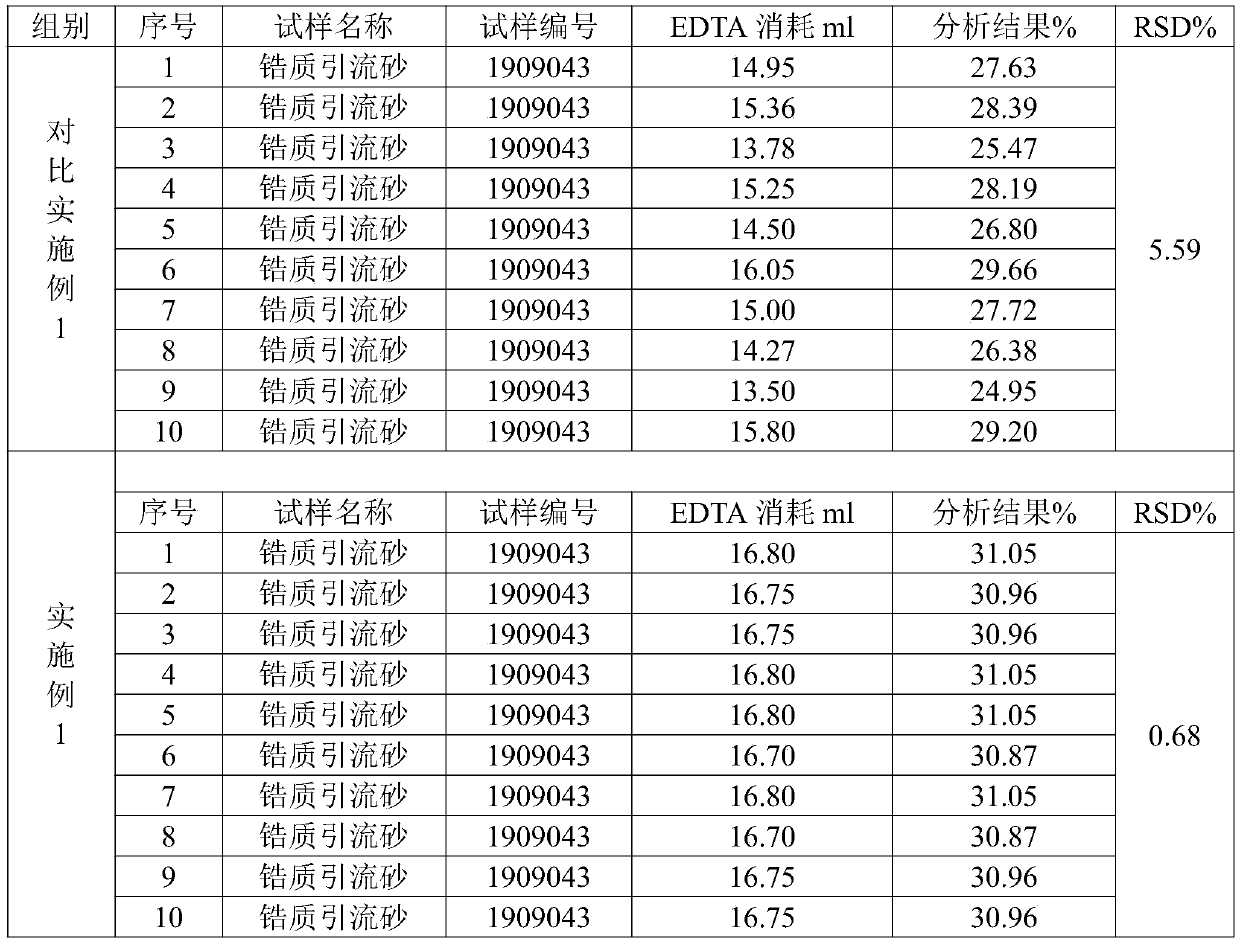
InactiveCN110346507ASolution is not easy to dissolveImprove accuracyAnalysis using chemical indicatorsChemical analysis using titrationZirconium hydrideMolten state
The invention provides a measurement method for zirconium oxide in drainage sand. The measurement method is characterized by comprising the following steps of preparing a reagent, preparing a test sample in a molten state, performing titration, and calculating zirconium oxide content. The measurement method for the zirconium oxide in the drainage sand has the beneficial effects that flux by whichthe zirconium oxide sample can be completely dissolved is used, accuracy detection data of the measurement method shows that the experiment recycling rate is 99-102%, and precision detection data shows that the RSD of the method is 0.68% and is remarkably higher than that of normal standard of which the RSD is 3%. By the method, the problem that the sample is difficult to dissolve during the measurement process of the zirconium oxide in the drainage sand is solved, so that the accuracy and the precision of a zirconium oxide element in the drainage sand measured by an EDTA complexometric titration method are remarkably improved, and the measurement method can be applicable to production and verification.
Owner:XINING SPECIAL STEEL
Method for detecting free bismuth in colloidal bismuth pectin or preparation containing colloidal bismuth pectin
ActiveCN109991184AAvoid product qualityStrong specificityChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorBismuth / pectinLinear relationship
The invention provides a method for detecting free bismuth in colloidal bismuth pectin or a preparation containing colloidal bismuth pectin. The method includes the following steps of: placing colloidal bismuth pectin or the preparation containing colloidal bismuth pectin in a plastic centrifuge tube, adding water to shake for no more than 1min, obtaining a colloidal solution with uniform dissolution, centrifuging the colloidal solution immediately, separating the supernatant, measuring the content of bismuth in the supernatant by complexometric titration or ultraviolet spectrophotometry, andcalculating the content of free bismuth in colloidal bismuth pectin or the preparation containing colloidal bismuth pectin. The method for detecting free bismuth in colloidal bismuth pectin or the preparation containing colloidal bismuth pectin, established by the invention, has the advantages of strong specificity, high accuracy, good repeatability, good linear relationship and high sensitivity,and can be used as a mass control method for free bismuth in colloidal bismuth pectin or the preparation containing colloidal bismuth pectin to effectively control the quality of products of colloidalbismuth pectin or the preparation thereof.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com