Method for measuring hexafluorophosphoric acid radical ion by amperometric titration
A technology of hexafluorophosphate and amperometric titration, applied in the direction of electrochemical variables of materials, etc., can solve the problems of affecting the accuracy of measurement results, long operation time of gravimetric method, interference measurement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 A method for measuring the hexafluorophosphate ion of lithium hexafluorophosphate by amperometric titration, the steps are:
[0017] (1) Prepare the standard solution of tetraphenylarsenic chloride: accurately measure 8.00ml and dilute it to 100ml with saturated NaCl solution, and use 0.02055mol / l standard KI 3 The solution was calibrated, and the potential change of the tetraphenyl arsenic chloride solution was measured with a PXS-215 ion activity meter. The titration reaction equation is:
[0018] (C 6 h 5 ) 4 As + +I 2 +I→(C 6 h 5 ) 4 AsI 3
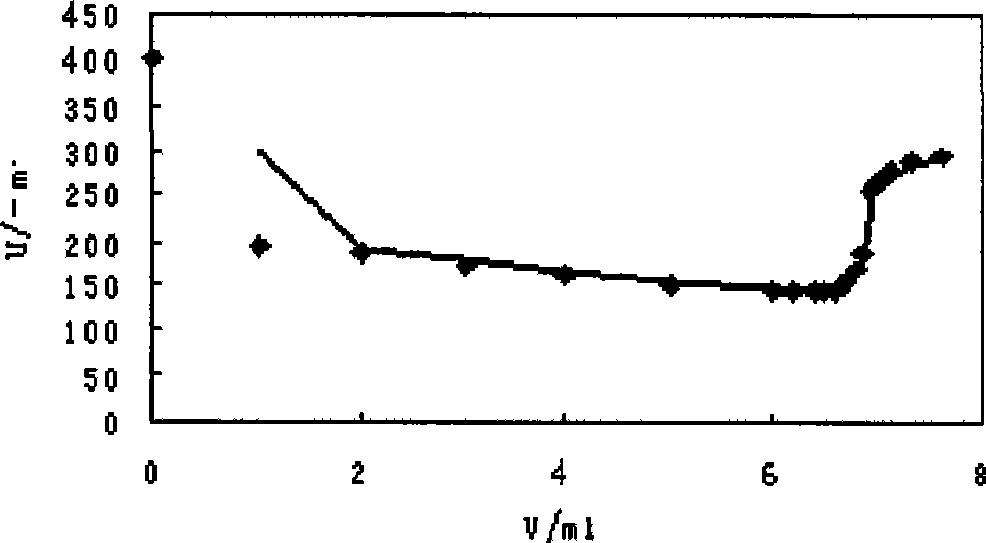
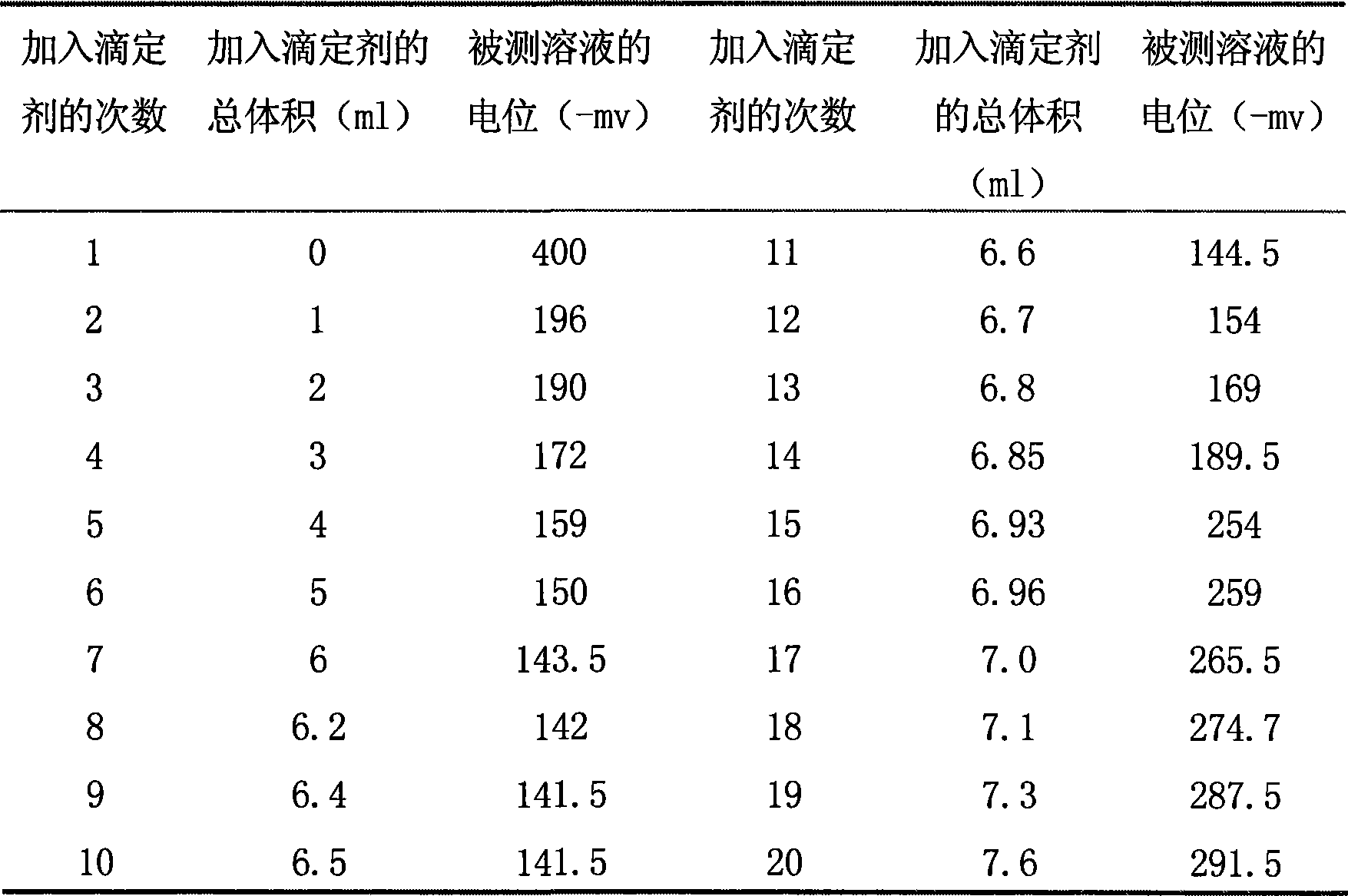
[0019] Calculate the end point of the titration, the volume of the standard potassium triiodide solution consumed and the concentration of the calibrated tetraphenyl arsenic chloride solution by using the second-order differential quotient method.
[0020] Calibrate the concentration of tetraphenylarsenic chloride solution.
[0021] The concentration titration data of tetraphenyl arsenic chloride solutio...
Embodiment 2
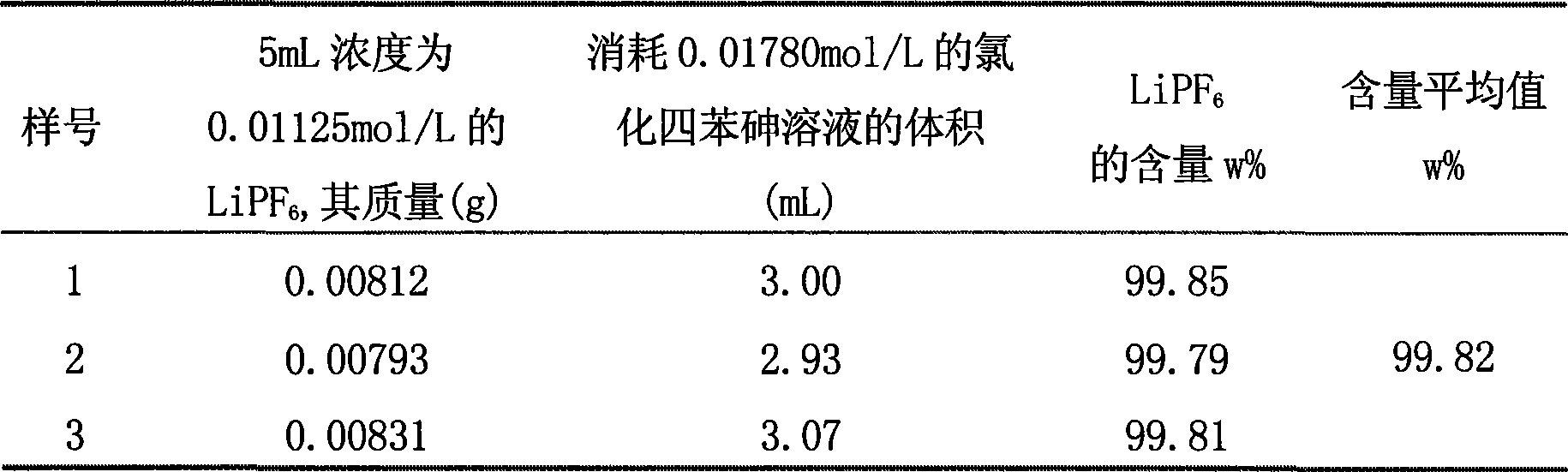
[0051] Select a newly-purchased commercial-grade lithium hexafluorophosphate sample (content 99.8%), adopt current polarography and gravimetric method to carry out comparative determination respectively, use tetraphenyl arsenic chloride as titrant to carry out current titration to hexafluorophosphate ion, the determined The results are shown in Table 2. Tetraphenylarsenic chloride was used as a precipitating agent to carry out gravimetric determination of hexafluorophosphate ions, and the results are shown in Table 3.
[0052] The results showed that the sample PF determined by the two methods - 6 The average value of the content is consistent, which shows that the current titration method of hexafluorophosphate ion using tetraphenylarsenic chloride as a titrant is a fast, accurate and economical analysis method.
[0053] Table 2 Amperometric titration of hexafluorophosphate ion with tetraphenylarsenic chloride as titrant
[0054]
[0055] Table 3 uses tetraphenylarsenic ...
Embodiment 3
[0059] In the research of preparing lithium hexafluorophosphate by solvent method, carry out 6 parallel determinations for the same sample of the lithium hexafluorophosphate product synthesized by oneself, calculate the standard deviation and relative standard deviation (R SD value), the test shows that the precision of the method can meet the requirements of analysis and determination. The measurement results are shown in Table 4.
[0060] Table 4 precision test results
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com