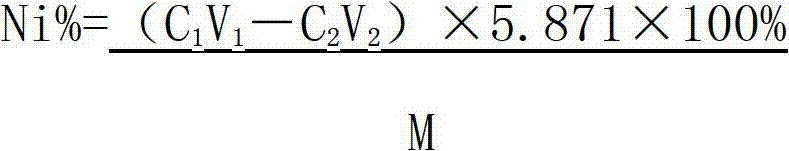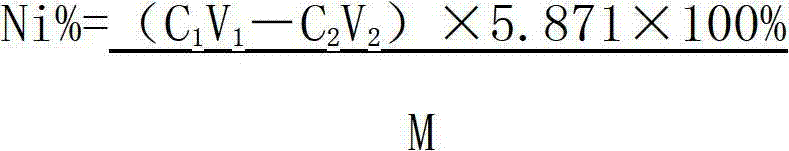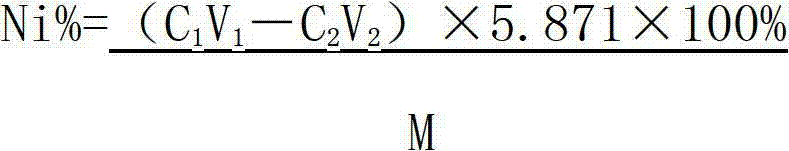Method determining content of nickel in nickel-titanium shape memory alloy
A memory alloy and nickel content technology, applied in the field of chemical analysis, can solve the problems of difficult observation of the titration end point, easy introduction of errors, long measurement cycle, etc., and achieve the effect of obvious mutation of the titration end point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This embodiment is a method for determining the nickel content in a titanium-nickel shape memory alloy, and the grade of the titanium-nickel shape memory alloy is TiNiss. This embodiment includes the following steps:
[0024] Step 1, sample weighing. Weigh 0.1g TiNiss sample and place it in a 250mL beaker. Obtain an accurately weighed TiNiss sample. The weight of the TiNiss sample to be weighed is accurate to 0.0001g.
[0025] Step 2, prepare the sample solution. Add 1 to 3 g of ammonium fluoride and 4 to 12 mL of pure nitric acid in sequence in a beaker, and dissolve it at 50 to 80 ° C to form a nitric acid solution, and the resulting nitric acid solution has no precipitates and impurities; in this example, the The dissolution temperature is 70°C. The wall of the beaker containing the obtained nitric acid solution was purged with distilled water in a conventional method, and the distilled water used for purging was mixed evenly with the nitric acid solution in the...
Embodiment 2
[0039] This embodiment is a method for the nickel content in a titanium-nickel shape memory alloy, and the grade of the titanium-nickel shape memory alloy is TiNiyy. This embodiment includes the following steps:
[0040] Step 1, sample weighing. Weigh 0.1g TiNiyy sample and place it in a 250mL beaker. Obtain an accurately weighed TiNiyy sample. The weight of the TiNiyy sample taken is accurate to 0.0001g.
[0041] Step 2, prepare the sample solution. Add 1 to 3 g of ammonium fluoride and 4 to 12 mL of pure nitric acid in sequence in a beaker, dissolve at 50 to 80 ° C to form a nitric acid solution, and the resulting nitric acid solution has no precipitates and impurities; in this example, the The dissolution temperature is 50°C. The wall of the beaker containing the obtained nitric acid solution was purged with distilled water in a conventional method, and the distilled water used for purging was mixed evenly with the nitric acid solution in the beaker to obtain a sample ...
Embodiment 3
[0055] This embodiment is a method for determining the nickel content in a titanium-nickel shape memory alloy, and the grade of the titanium-nickel shape memory alloy is TiNi-01. This embodiment includes the following steps:
[0056] Step 1, sample weighing. Weigh 0.1g TiNi-01 sample and place it in a 250mL beaker. Accurately weighed TiNi-01 samples were obtained. The weight of the TiNi-01 sample taken by weighing is accurate to 0.0001g.
[0057] Step 2, prepare the sample solution. Add 1 to 3 g of ammonium fluoride and 4 to 12 mL of pure nitric acid in sequence in a beaker, dissolve at 50 to 80 ° C to form a nitric acid solution, and the resulting nitric acid solution has no precipitates and impurities; in this example, the The dissolution temperature is 80°C. The wall of the beaker containing the obtained nitric acid solution was purged with distilled water in a conventional method, and the distilled water used for purging was mixed evenly with the nitric acid solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com