Method for testing constant cadmium and zinc in cadmium zinc telluride crystal
A test method, cadmium zinc telluride technology, applied in the field of chemical analysis, can solve the problems of interfering titration end point observation, inability to perform accurate titration, insensitive end point discoloration, etc., to reduce analysis cost, reduce relative standard deviation, and suppress interference Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Determination of constant cadmium and zinc content in standard samples:
[0042] (1) Accurately weigh 1.1364g of 99.999% cadmium powder, add 20mL of nitric acid (1+1) and heat to dissolve in the conical flask, accurately weigh 1.3336g of 99.999% tellurium powder, add 20mL of nitric acid (1+1 1) Heat and dissolve in the Erlenmeyer flask, accurately weigh 0.6538g of 99.999% zinc flakes, dissolve in the Erlenmeyer flask with a small amount of hydrochloric acid, transfer the dissolved zinc solution together with the cooled cadmium solution and tellurium solution to Add 60mL of concentrated nitric acid to a 1L volumetric flask, dilute to the mark with pure water, and reserve it as the standard solution for CZT synthesis.
[0043](2) Use a pipette to accurately pipette 25mL of CZT-synthesized standard solution into a 250mL Erlenmeyer flask, adjust to near-neutrality with ammonia water (1+1), at this time a large amount of white precipitate is produced, and then add 15mL of am...
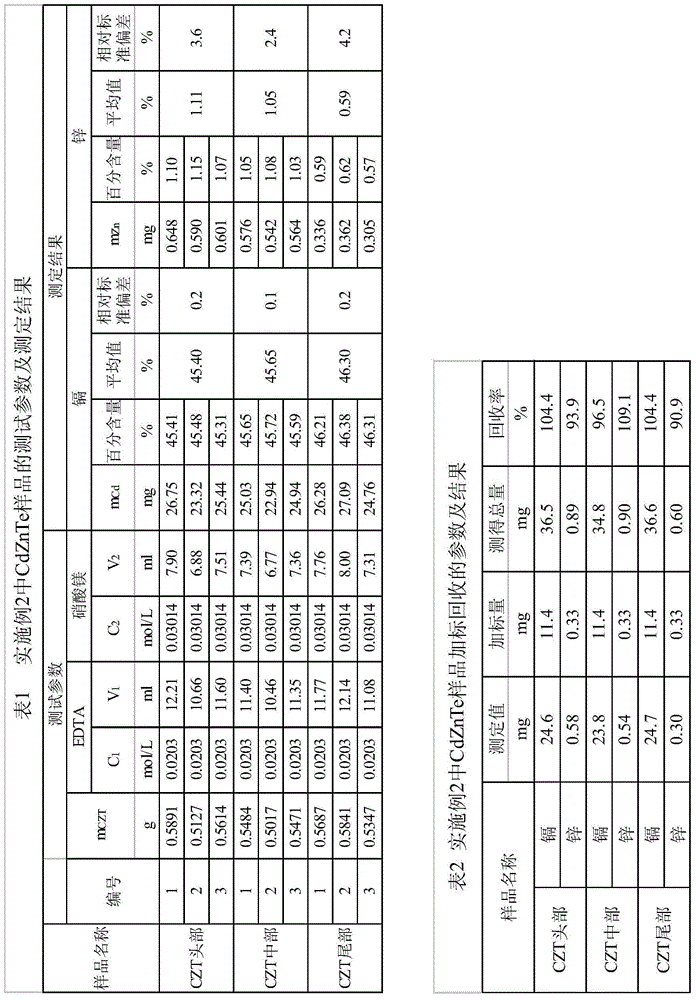
Embodiment 2
[0059] Determination of cadmium and zinc content in CZT crystal produced by our company:
[0060] (1) Accurately weigh 0.5g~0.6g (accurate to 0.0001g) of CdZnTe sample in a conical flask, add 10mL of nitric acid (1+1), heat at low temperature until completely dissolved, transfer the solution to a 250mL flask after cooling Add 10mL of nitric acid (1+1) to the volumetric flask, dilute to the mark with pure water and shake well.
[0061] (2) Use a pipette to accurately pipette 25mL of the sample solution, adjust the pH value to near neutrality with ammonia water (1+1), at this time a large amount of white precipitate is produced, then add 15mL ammonia water-ammonium chloride buffer solution to maintain the pH of the solution The value is 10.
[0062] (3) Add 5 mL of polyethylene glycol octyl phenyl ether surfactant with a volume concentration of 20% to the solution. At this time, the white precipitate disappears. Shake well to dissolve it fully, and then add 0.05 g of chrome bla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com