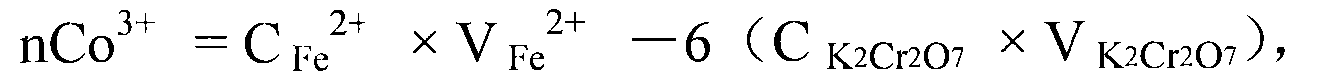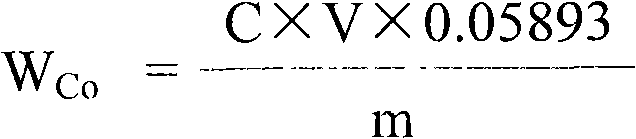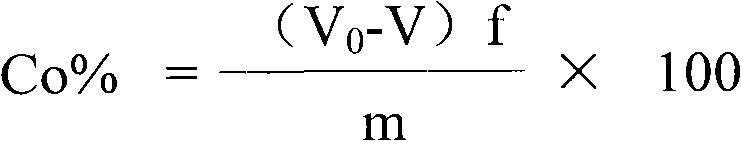Measuring method of divalent cobalt content in lithium cobalt oxide
A technology of lithium cobalt oxide and its determination method, which is applied in the direction of material analysis through observation of the influence on chemical indicators, and analysis through chemical reaction of materials, and can solve the problem of lack of determination of divalent cobalt content in lithium cobalt oxide Methods and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The assay method of the divalent cobalt content in the lithium cobalt oxide of this example is to measure the total cobalt content and the trivalent cobalt ion content in the lithium cobalt oxide respectively, then subtract the trivalent cobalt ion content from the total cobalt content to obtain the lithium cobalt oxide Divalent cobalt content in lithium cobalt oxide; wherein, the assay method of total cobalt content in lithium cobalt oxide adopts ethylaminetetraacetic acid (EDTA) complexometric volumetric method; The assay method of trivalent cobalt ion content in lithium cobalt oxide adopts ferrous sulfate Ammonium redox titration method, the steps are as follows:
[0041] 1. Total cobalt content in lithium cobalt oxide
[0042] 1.1 Main reagents
[0043] Cobalt standard solution 0.05mol / L: Weigh 2.9500g of cobalt metal sheet (purity 99.98%), put it in a 250ml beaker, add a small amount of hydrochloric acid (P=1.19g cm -3 ) and hydrogen peroxide (30%, percent by wei...
Embodiment 2
[0088] The determination method of divalent cobalt content in the lithium cobalt oxide of this example adopts the iodometric method except that the determination method of the total cobalt content in the lithium cobalt oxide is the same as embodiment one.
[0089] The assay steps of total cobalt content in the lithium cobalt oxide of this example are as follows:
[0090] 1. Method summary:
[0091] co 2+ It can be oxidized to Co by iodine in ammoniacal solution (PH=9~10) containing ammonium nitrate 3+ , and generate stable green precipitate of nitric acid-iodine pentaammine cobalt with iodine. Excess iodine was titrated with standard sodium arsenite solution using starch as indicator. Its reaction formula is as follows:
[0092] 2Co 2+ +4NO 3 - +10NH 3 +I 2 →2〔Co(NH 3 ) 5 I〕(NO 3 ) 2 ↓
[0093] I 2 +AsO 3 3- +H 2 O→AsO 4 3- +2HI
[0094] Iron and aluminum can form hydroxide precipitates in ammonia solution and easily adsorb cobalt. At the same time, iron h...
Embodiment 3
[0110] The determination method of the divalent cobalt content in the lithium cobalt oxide of this example is the same as the first embodiment except that the determination method of the total cobalt content in the lithium cobalt oxide adopts the potassium ferricyanide redox titration method.
[0111] The assay steps of total cobalt content in the lithium cobalt oxide of this example are as follows:
[0112] 1. Method summary
[0113] In ammoniacal solution, the Co 2+ Oxidized to Co 3+ , an appropriate amount of potassium ferricyanide with Co 2+ Standard solution back titration.
[0114] co 2+ +【Fe(CN) 6 】 3- →Co 3+ +【Fe(CN) 6 】 4-
[0115] Under the conditions of this Act, Fe 2+ Easily oxidized to Fe under ammonia conditions 3+ , interference determination. However, adding ammonium citrate and ammonium sulfate to the mixed solution with ammonia water can eliminate interference.
[0116] 2 Instruments and main reagents
[0117] 2.1 ZD-2 type automatic potentiome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com