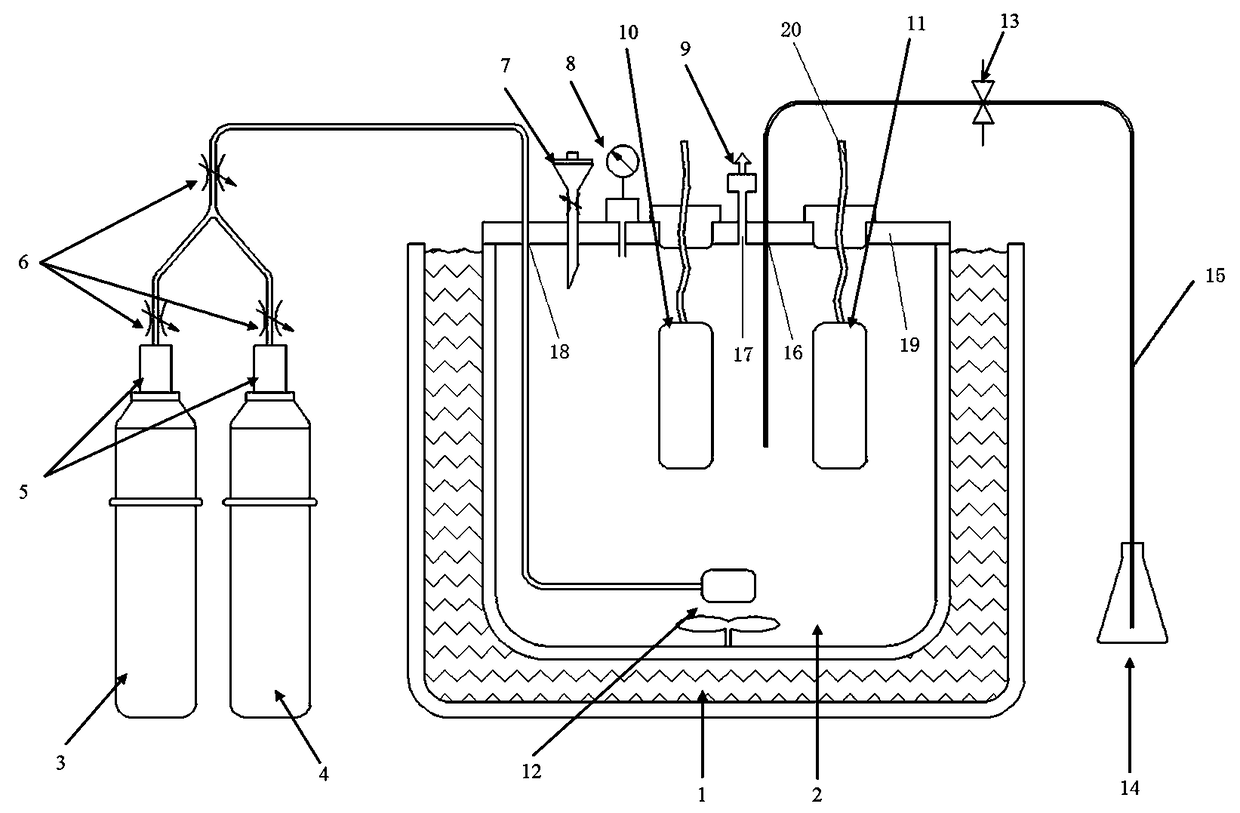
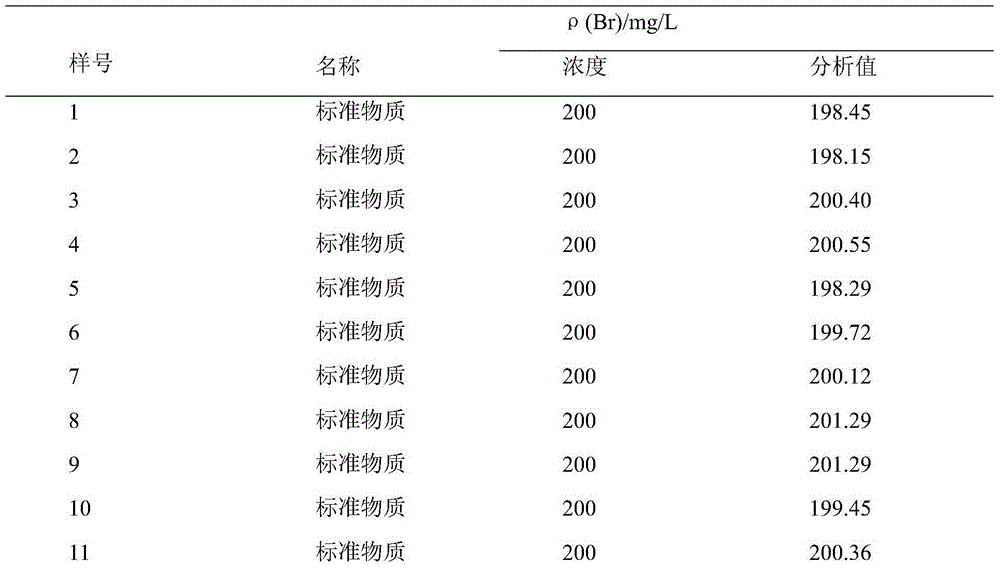
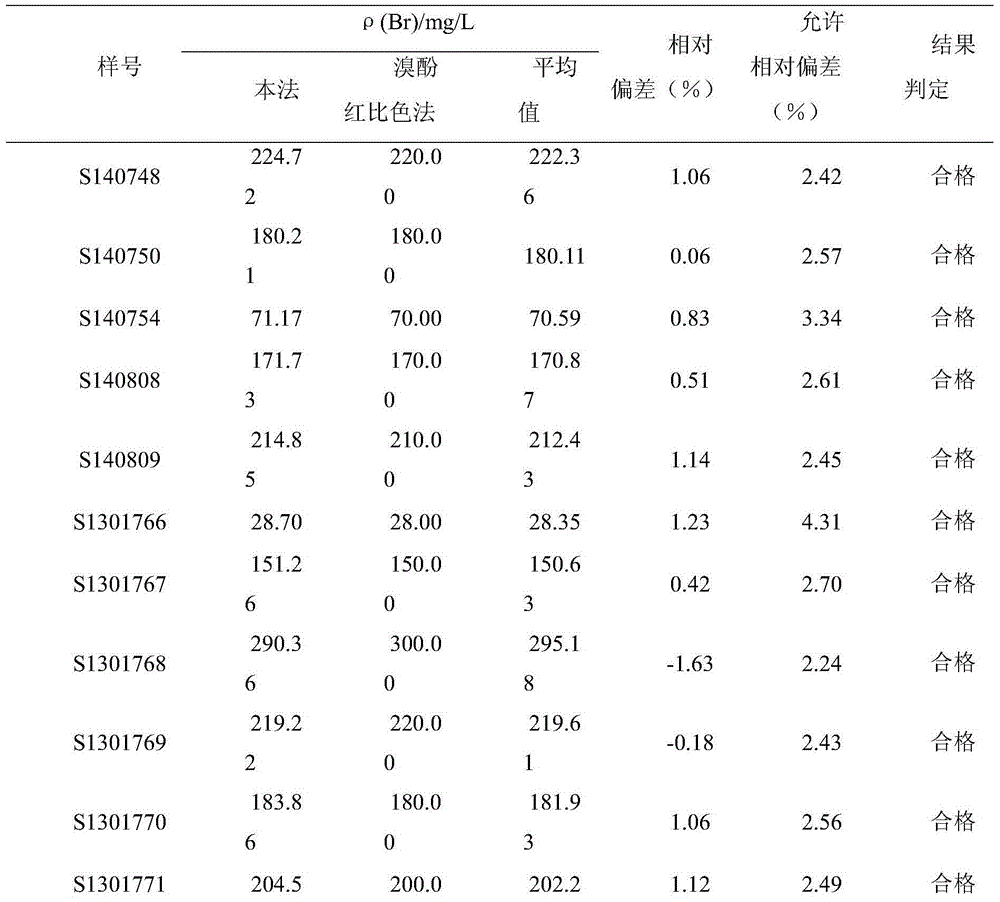
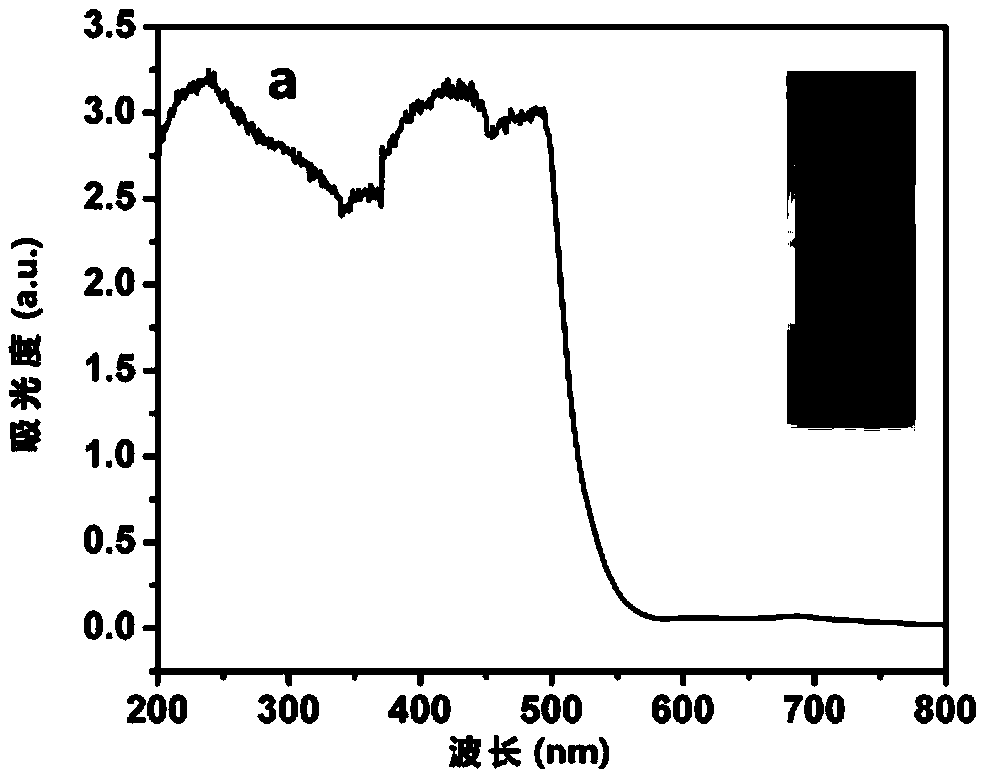
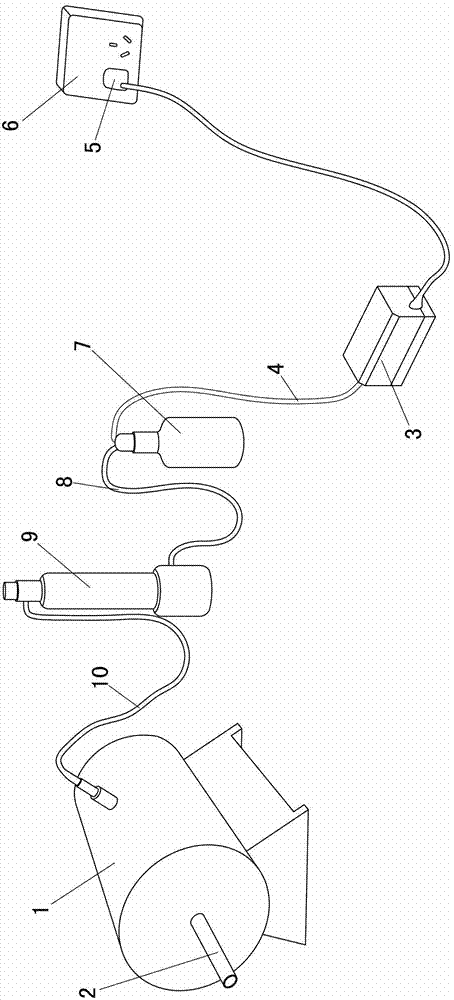
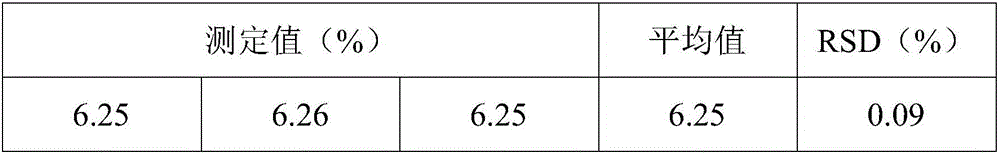
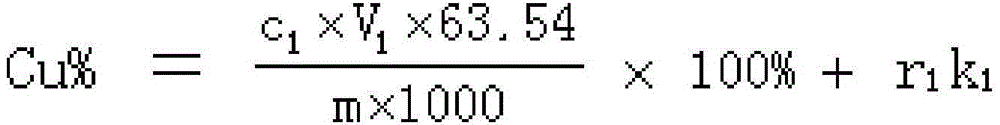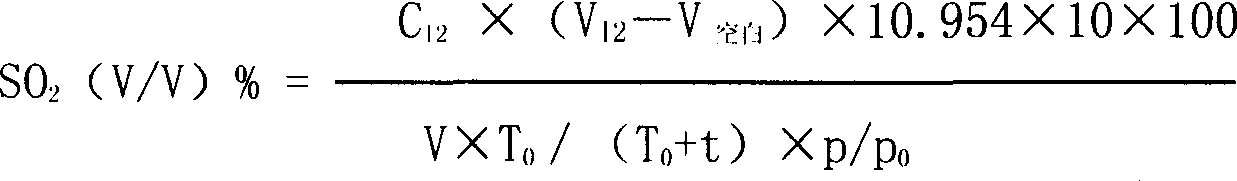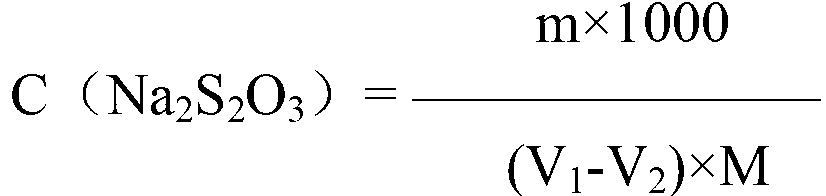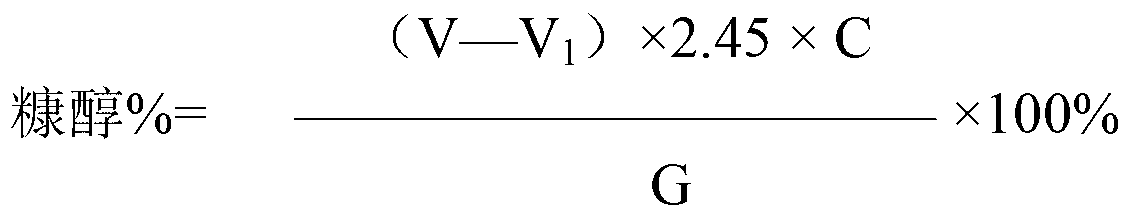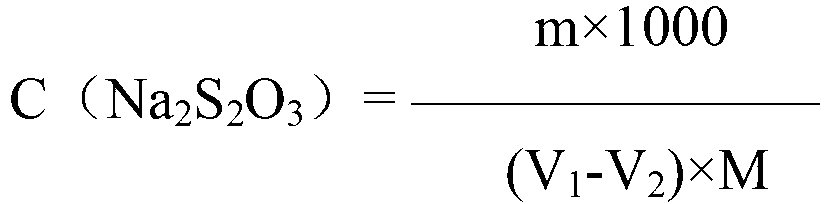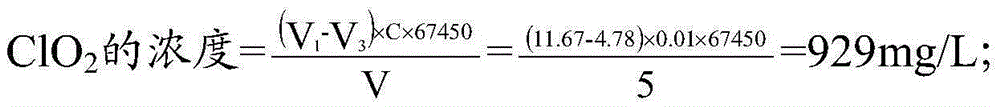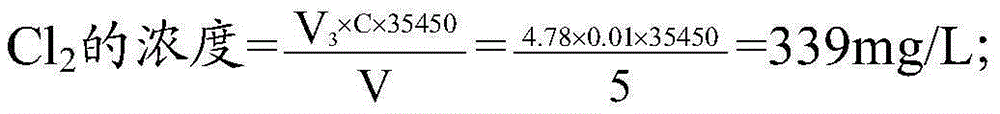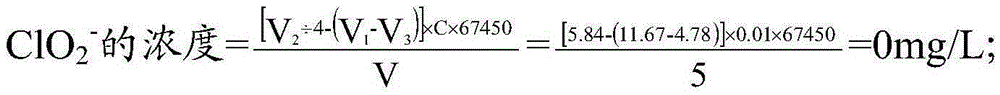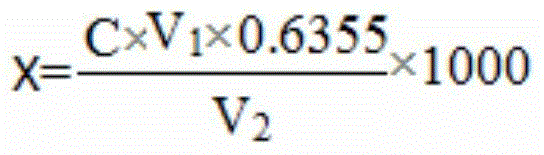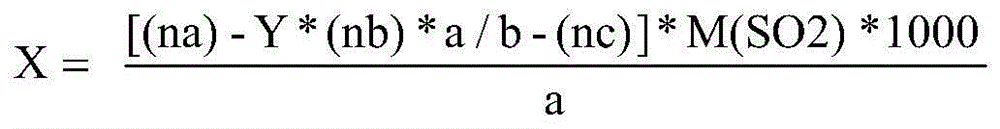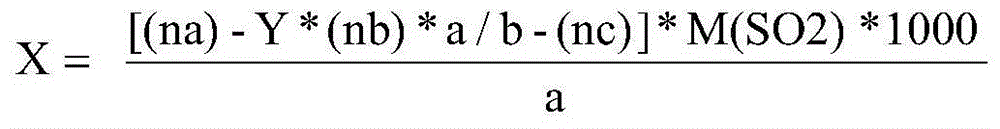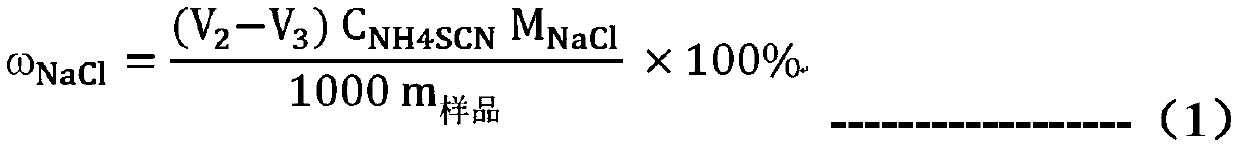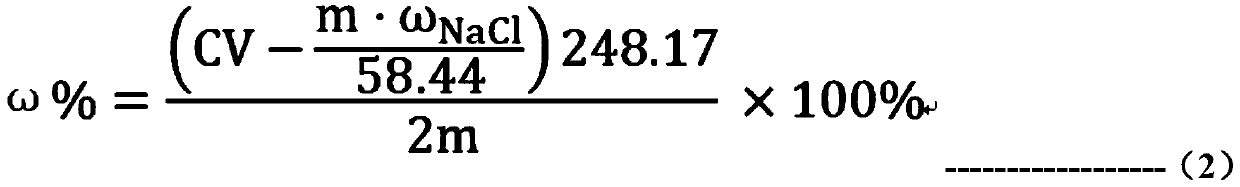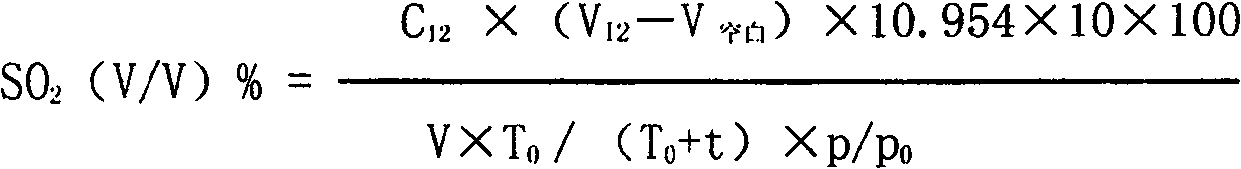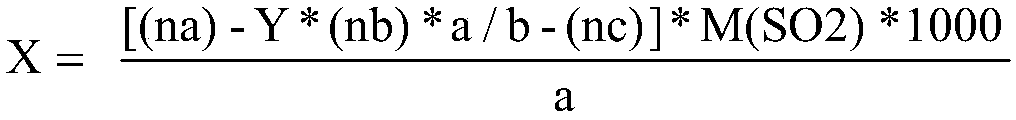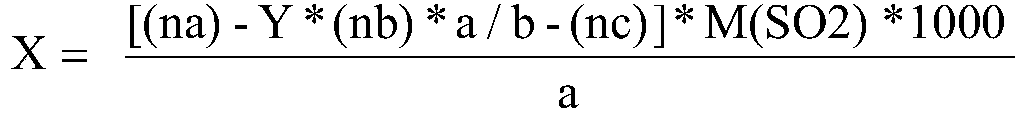Patents
Literature
48 results about "Iodometric method" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Iodometry, also known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point.
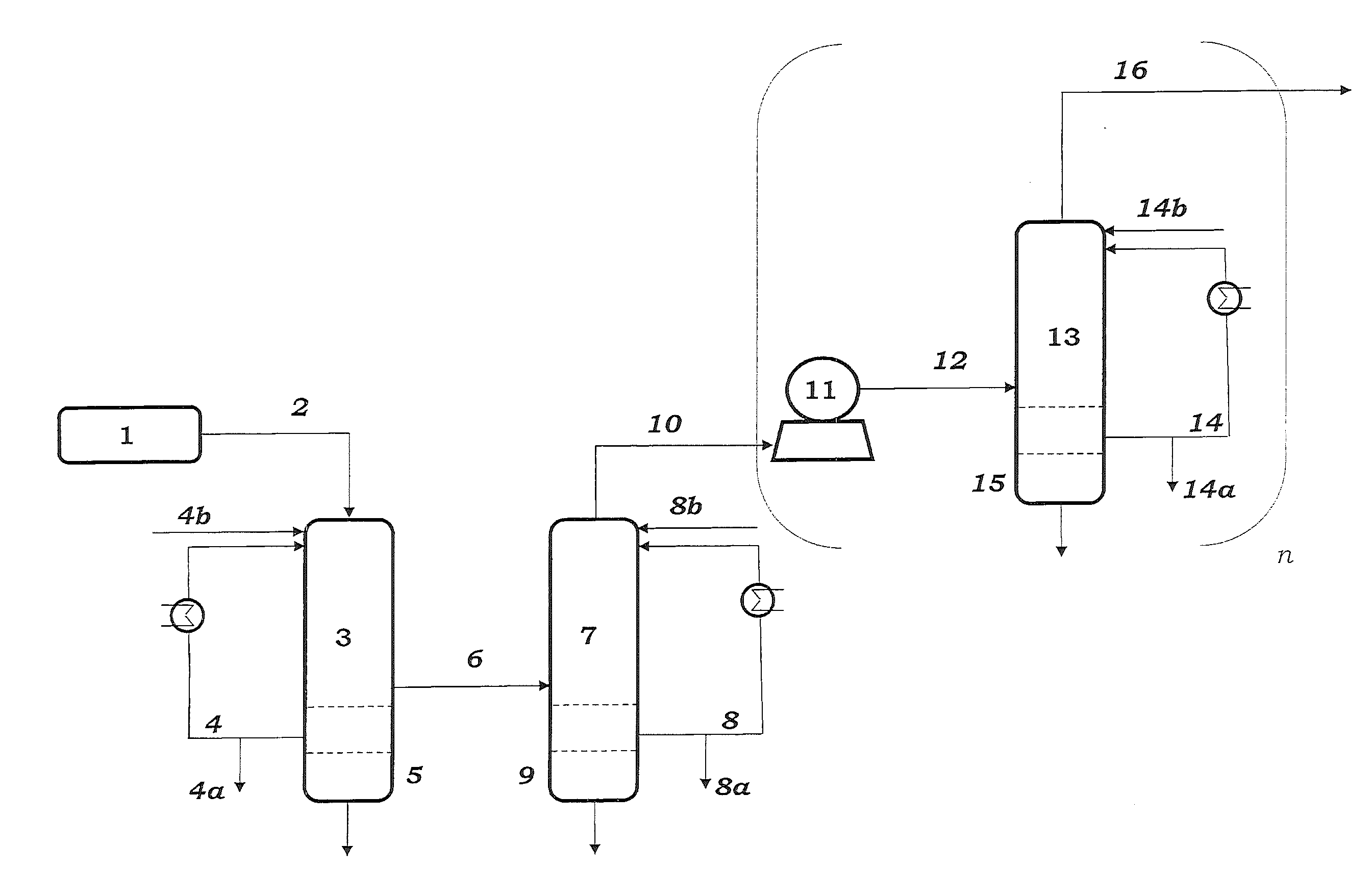
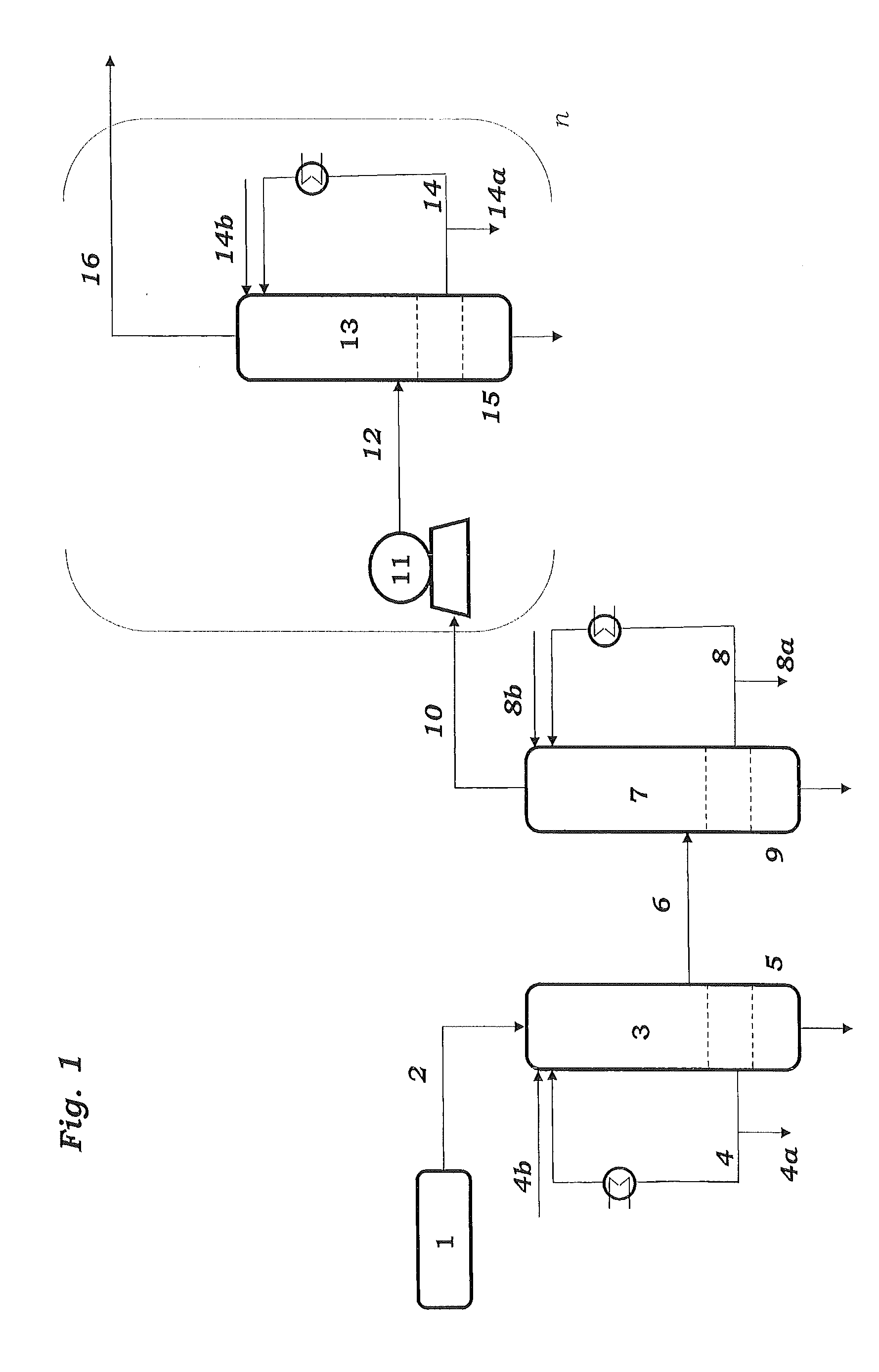
Process for Preparing Butadiene by Oxidative Dehydrogenation of N-Butenes with Monitoring of the Peroxide Content During Work-Up of the Product
InactiveUS20140200381A1Analysis using chemical indicatorsHydrocarbon by hydrogenationWater vaporDesorption
The invention relates to a process for preparing butadiene from n-butenes, which comprises the following steps:A) provision of a feed gas stream a comprising n-butenes;B) introduction of the feed gas stream a comprising n-butenes and an oxygen-comprising gas into at least one dehydrogenation zone and oxidative dehydrogenation of n-butenes to butadiene, giving a product gas stream b comprising butadiene, unreacted n-butenes, water vapor, oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases;C) cooling and compression of the product gas stream b in at least one cooling stage and at least one compression stage, with the product gas stream b being brought into contact with a circulated coolant to give at least one condensate stream c1 comprising water and a gas stream c2 comprising butadiene, n-butenes, water vapor, oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases;D) separation of incondensable and low-boiling gas constituents comprising oxygen, low-boiling hydrocarbons, possibly carbon oxides and possibly inert gases as gas stream d2 from the gas stream c2 by absorption of the C4-hydrocarbons comprising butadiene and n-butenes in a circulated absorption medium, giving an absorption medium stream loaded with C4-hydrocarbons and the gas stream d2, and subsequent desorption of the C4-hydrocarbons from the loaded absorption medium stream to give a C4 product gas stream d1;E) separation of the C4 product stream d1 by extractive distillation using a solvent which is selective for butadiene into a stream e1 comprising butadiene and the selective solvent and a stream e2 comprising n-butenes;F) distillation of the stream e1 comprising butadiene and the selective solvent to give a stream f1 consisting essentially of the selective solvent and a stream f2 comprising butadiene;where samples are taken from the circulated coolant in step C) and / or the circulated absorption medium in step D) and the peroxide content of the samples taken is determined by means of iodometry, differential scanning calorimetry (DSC) or microcalorimetry.
Owner:BASF AG
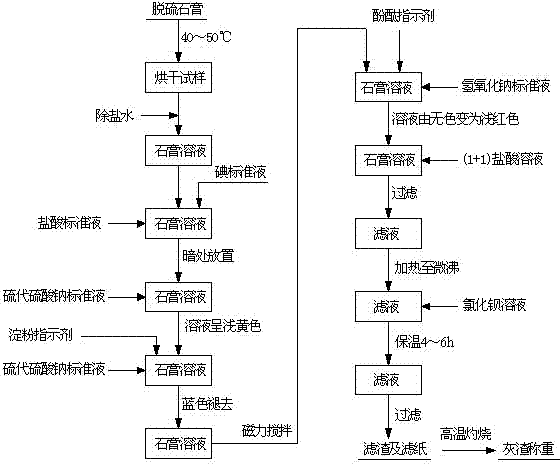
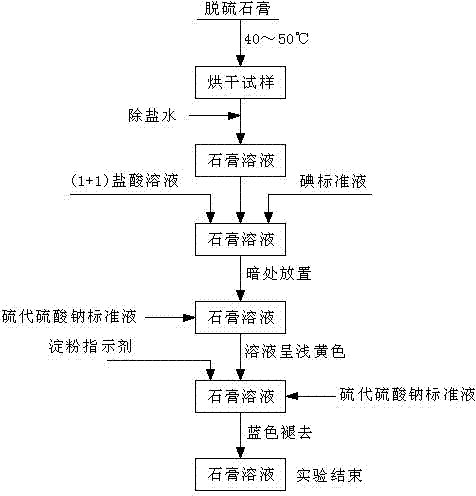
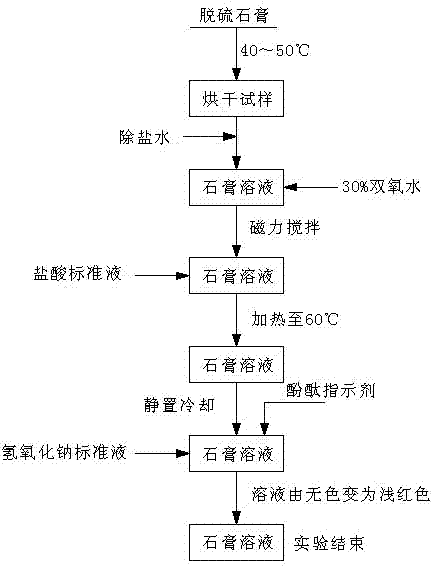
Process for continuously and rapidly determining components in desulfurization gypsum
ActiveCN102331422AReduce typesReduce dosageWeighing by removing componentChemical analysis using titrationIodometric methodPhysical chemistry
The invention which relates to a process or continuously and rapidly determining components in desulfurization gypsum belongs to the field of chemical detection. The process adopts iodometry, a back titration method with sodium hydroxide and a gravimetric method with barium sulfate in sequence to determine. The process of the invention has the advantages of reasonable design of test steps, easy and laborsaving operation, low cost, less pollution, high accuracy and less test workload.
Owner:HUADIAN ELECTRIC POWER SCI INST CO LTD
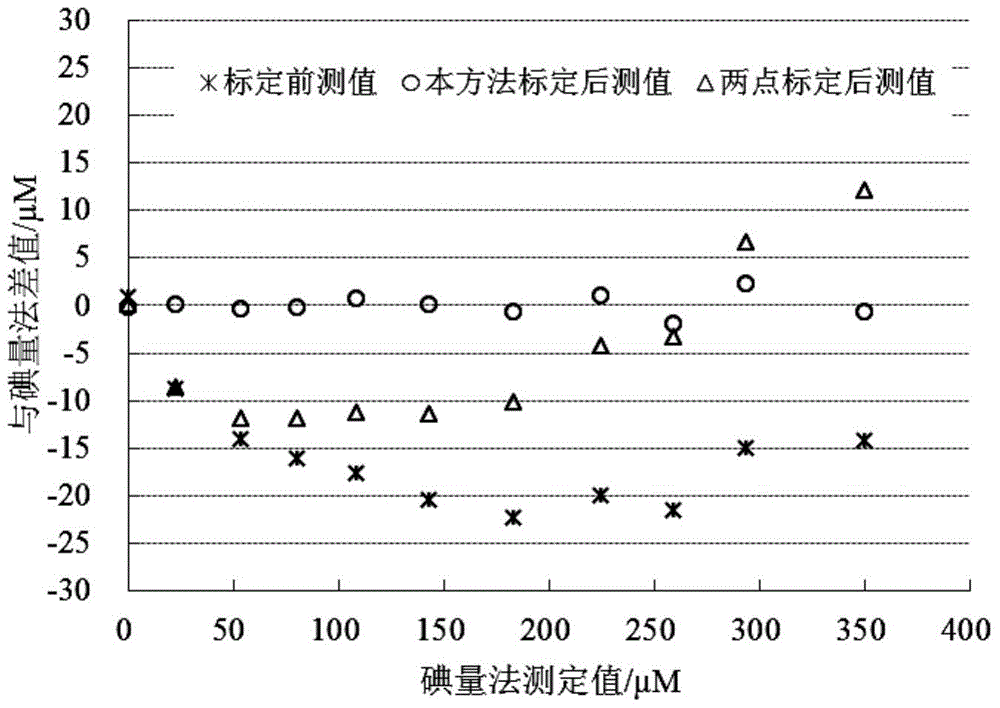
High-precision optical dissolved oxygen sensor calibration method and device
ActiveCN104515761AFlexible settingsBaseline Accuracy ImprovementFluorescence/phosphorescenceSolubilityConcentration gradient
Owner:SECOND INST OF OCEANOGRAPHY MNR
Method for preparing enzyme electrode and rapidly detecting peroxide value of vegetable oil
InactiveCN102435650BHigh sensitivityHigh precisionMaterial analysis by electric/magnetic meansVegetable oilHorse radish peroxidase
The invention belongs to the field of food detection, and particularly relates to a method for preparing an enzyme electrode and rapidly detecting the peroxide value of vegetable oil. The method comprises the steps of: selecting a glassy carbon electrode for use; firstly preparing a Nafion-methylene blue membrane electrode, then adopting a bovine serum albumin-glutaraldehyde crosslinking method to fix horse radish peroxidase (HRP), and obtaining a final HRP electrode; and then using the HRP electrode to rapidly detect the peroxide value of the vegetable oil. The method is high in sensitivity, good in selectivity, high in accuracy, short in response time, less in interference and superior to the traditional iodometry, thus being a simple, rapid, convenient and easy method for measuring the peroxide value and having the potential of realizing automatic on-site measurement.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
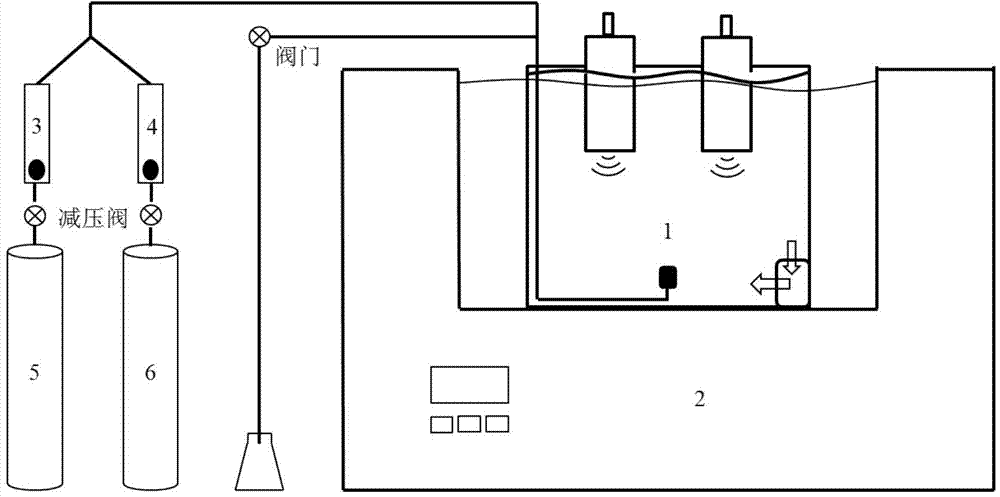
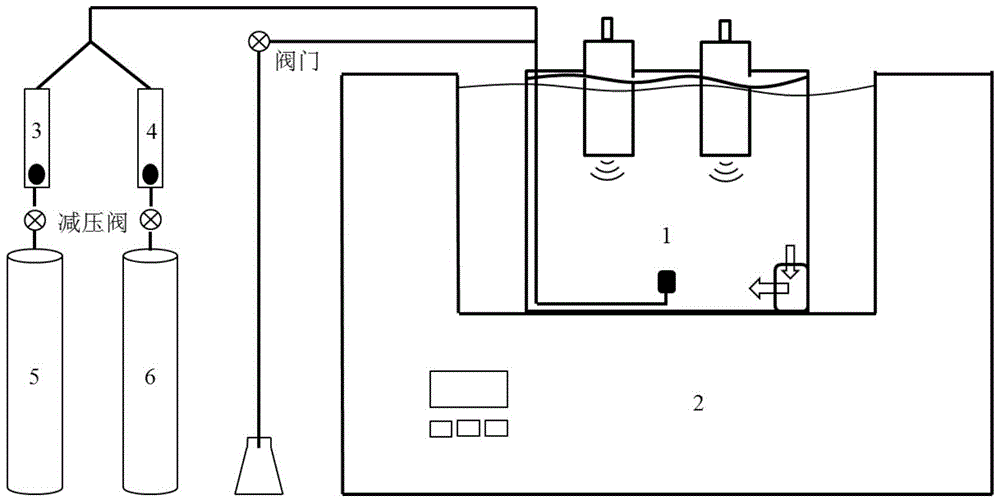
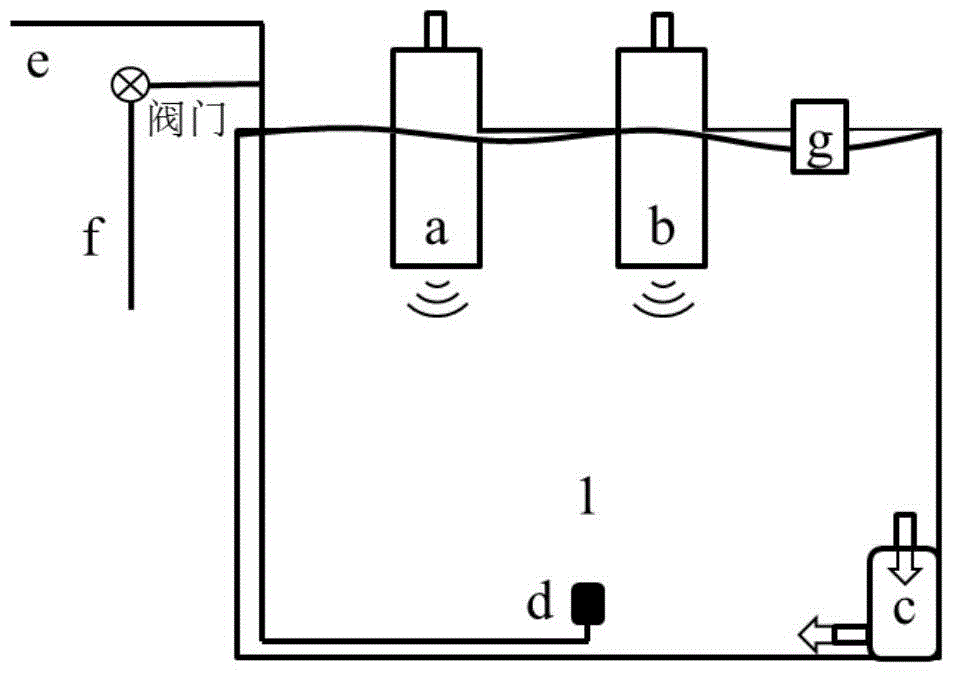
Multi-parameter disturbance compensating and correcting system and method of optical dissolved oxygen sensor
ActiveCN108663347AEffective control conditionsEasy to controlFluorescence/phosphorescenceIodometric methodOxygen sensor
The invention belongs to the technical field of dissolved oxygen sensor compensation and calibration, and discloses a multi-parameter disturbance compensating and correcting system of an optical dissolved oxygen sensor. The multi-parameter disturbance compensating and correcting system comprises a correcting tank, a watertight plug connector connected with the correcting tank, a gas lead-in device, a to-be-corrected dissolved oxygen sensor, a reference dissolved oxygen sensor, a sampling device, a salinity regulating device and a pressure regulating device, wherein the salinity regulating device and the pressure regulating device are arranged on the watertight plug connector. According to the technical scheme adopted by the invention, a gas mixture of different oxygen content is led into the correcting tank in sequence under different water body temperatures, different salinities and different environmental pressures, so that the water body has multiple dissolved oxygen concentrations,the phase value of the to-be-corrected dissolved oxygen sensor is recorded, and environmental parameters of the water body are recorded; the dissolved oxygen standard value is measured by taking a water sample through an iodometric method to calculate a multi-parameter disturbance compensating and correcting coefficient of the to-be-corrected dissolved oxygen sensor, so that automation degree isimproved, sensor calibrating precision, correcting precision and in-situ measuring accuracy are improved, and therefore, the sensor has a wider range of application.
Owner:OCEANOGRAPHIC INSTR RES INST SHANDONG ACAD OF SCI
Method for measuring bromine in brine through filter paper method sample preparation and X-ray fluorescent spectrometry
InactiveCN104483338ASolution rangeSolve the errorMaterial analysis using wave/particle radiationPreparing sample for investigationEnvironmental resistanceFluorescence
The invention relates to a method for measuring bromine in brine through filter paper method sample preparation and X-ray fluorescent spectrometry. Appropriate filter paper is selected for sample preparation, a standard curve is drawn with X-ray fluorescent spectrometry, and the content of bromine in brine is measured through comparison of sample tests. By means of the method for measuring bromine in brine through filter paper method sample preparation and X-ray fluorescent spectrometry, main problems of narrow linearity range, large errors, poor accuracy and precision and the like of a conventional bromine analytical test method are solved, and the method is fast, simple and convenient; compared with the conventional method, the analytical test efficiency is improved by 2-3 times, the analysis cost is low, and the material cost is 10% of that consumed in a sodium hypochloriteoxidation and iodometric method; the testing accuracy is high, the precision is good, the method detection limit is low, and the analysis requirement is met; and besides, the method doesn't need chemical reagent treatment, is particularly suitable for measuring a liquid with bromine content larger than 0.95 mg / L, realizes efficient and environment-friendly bromine measurement and is a real green analysis method.
Owner:山东省第四地质矿产勘查院
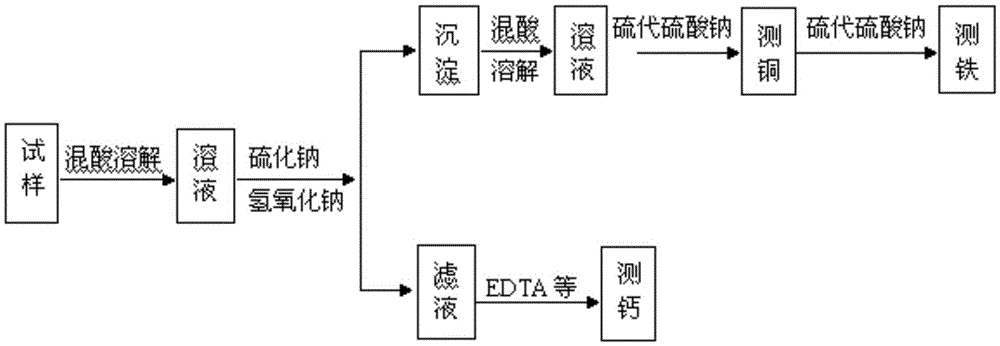
Method for detecting copper, iron and calcium in flash converting furnace slag
InactiveCN105467068AThe detection method is simpleEasy to operateChemical analysis using titrationEthylenediamineSlag
The invention provides a method for detecting copper, iron and calcium in flash converting furnace slag. The method comprises the following steps: (A) dissolving a flash converting furnace slag test sample with acid to obtain a first solution containing copper ions, iron ions and calcium ions, performing reaction on the first solution, sodium sulfide and sodium hydroxide, and filtering to obtain precipitates and filtrate; (B) dissolving the obtained precipitates with acid to obtain a second solution containing bivalent copper ions and iron ions, masking iron with ammonium bifluoride in the second solution, detecting copper by adopting an iodometry method to obtain the copper content, releasing iron in the solution subjected to copper detection with aluminium trichloride, and detecting iron by adopting the iodometry method to obtain the iron content; (C) titrating the filtrate obtained in the step (A) with edetic acid, thus obtaining the calcium content. The procedures of the detection method provided by the invention are simple, one-step sample weighing is realized, and the contents of three key elements, copper, iron and calcium, in the flash converting furnace slag can be quickly, accurately and continuously measured.
Owner:YANGGU XIANGGUANG COPPER
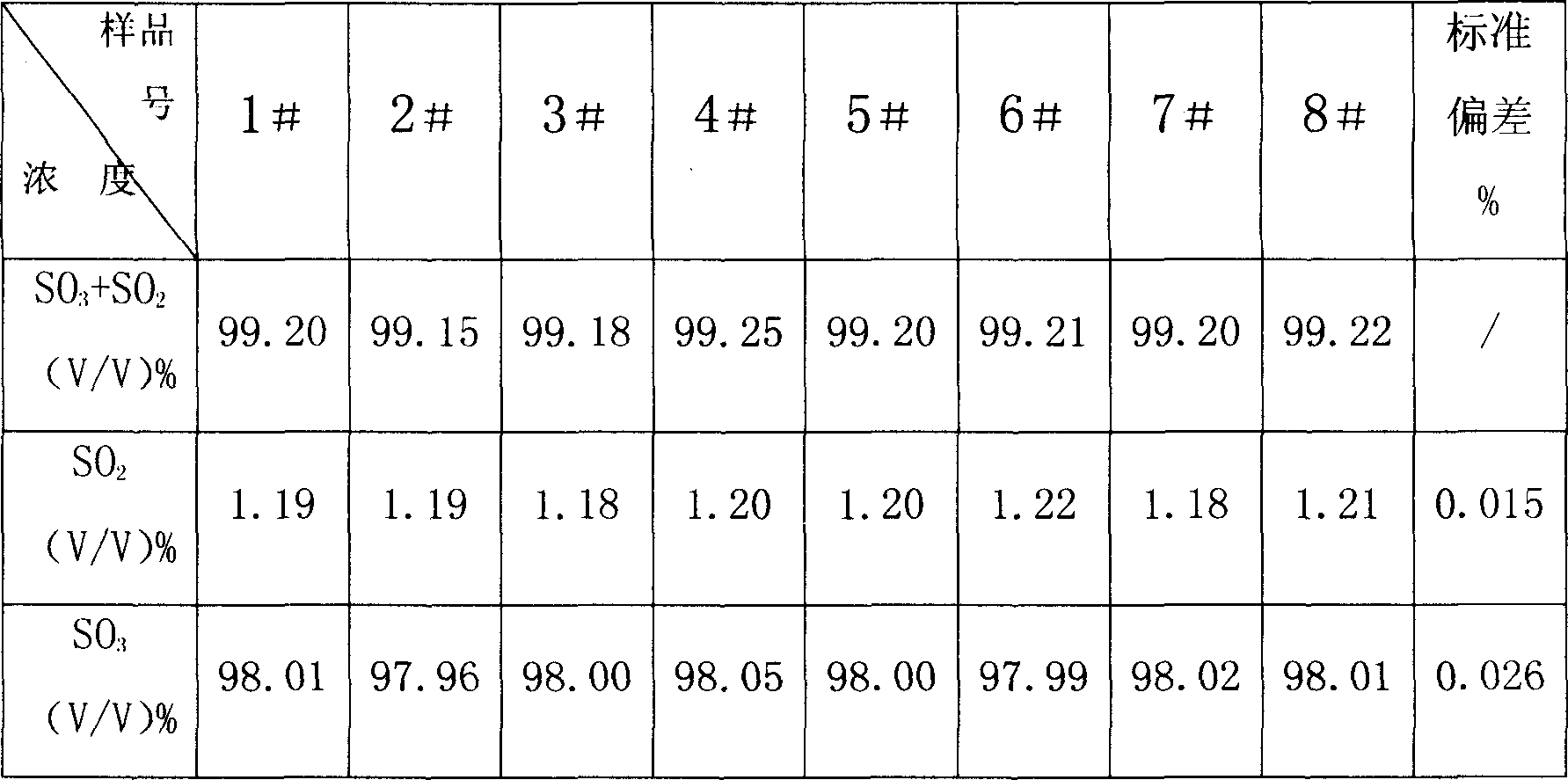
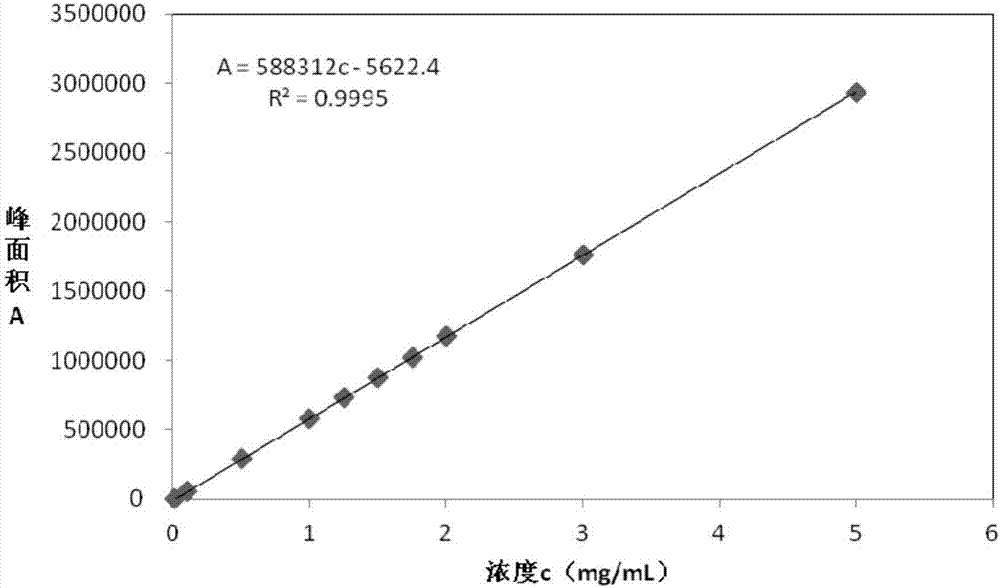
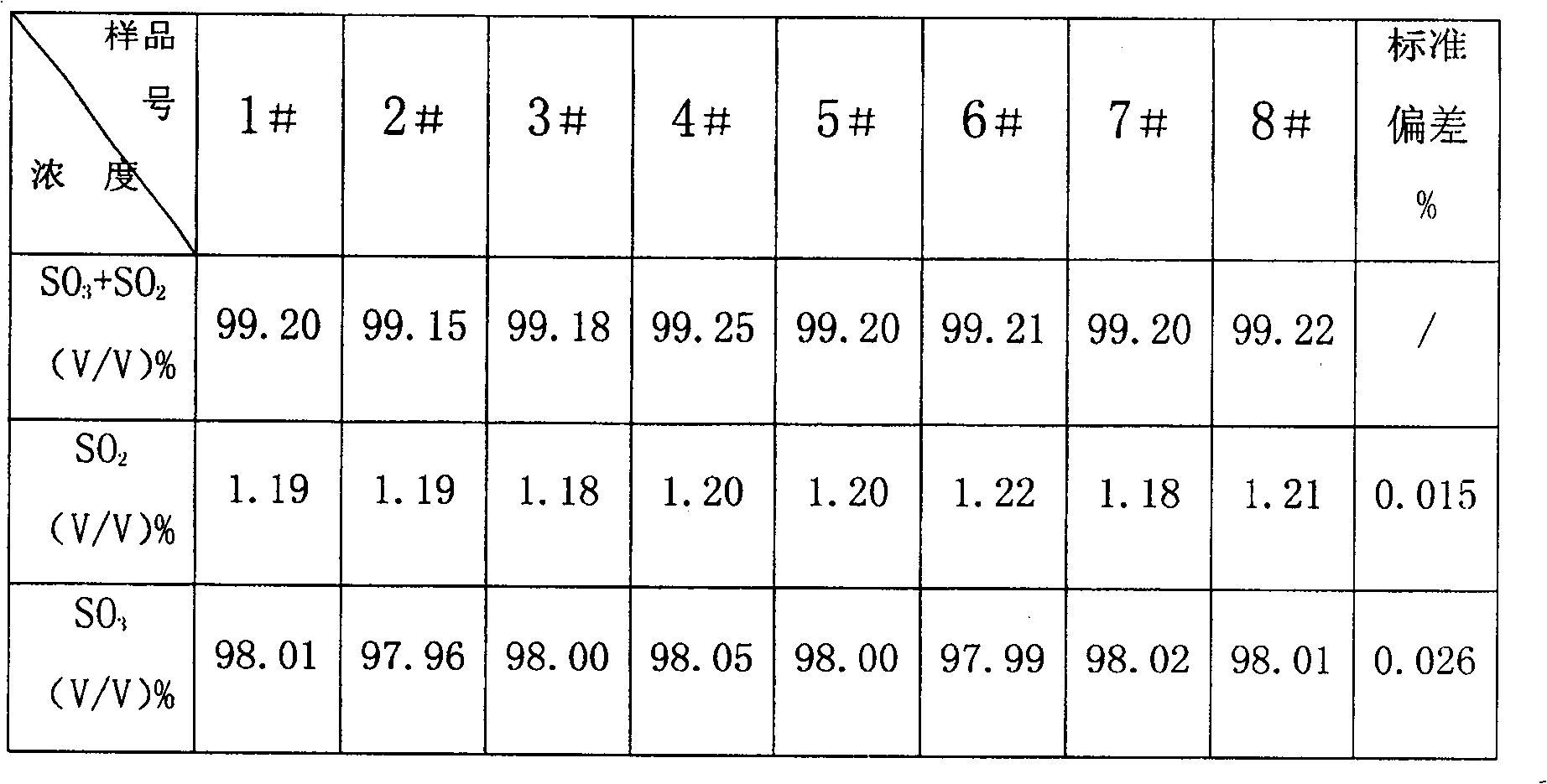
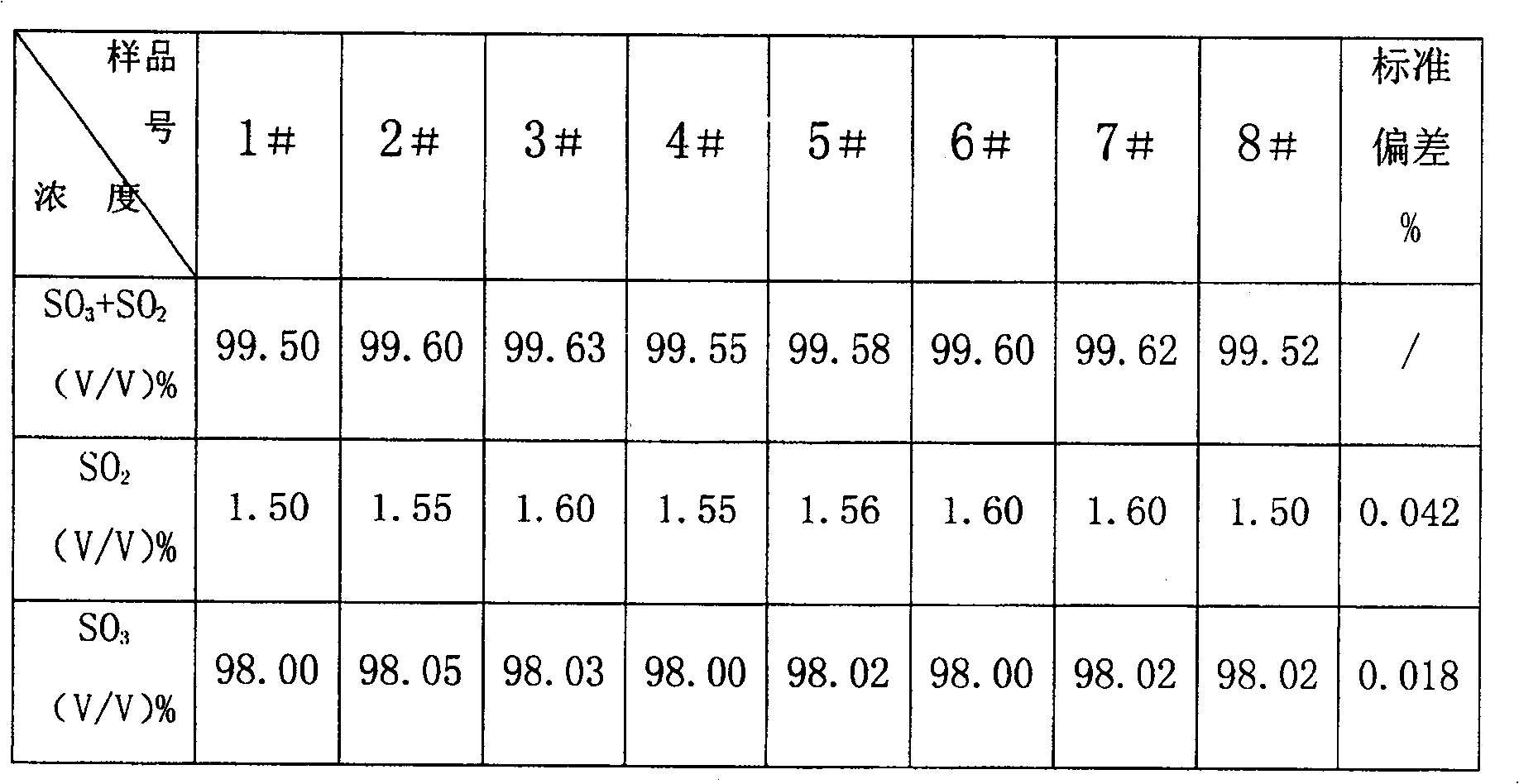
Combined detection method for high-purity SO3 gas and impurity SO2 gas therein
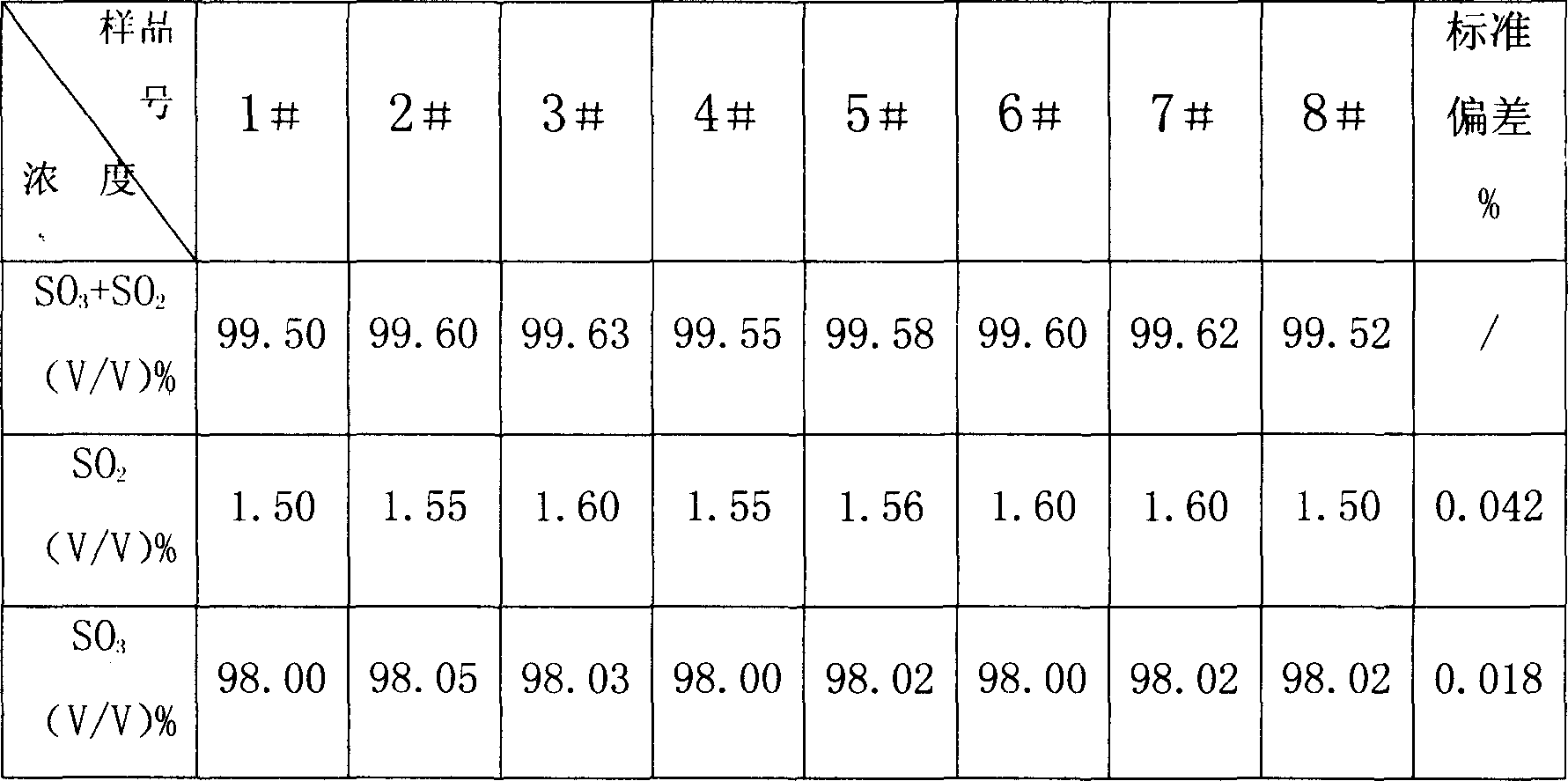
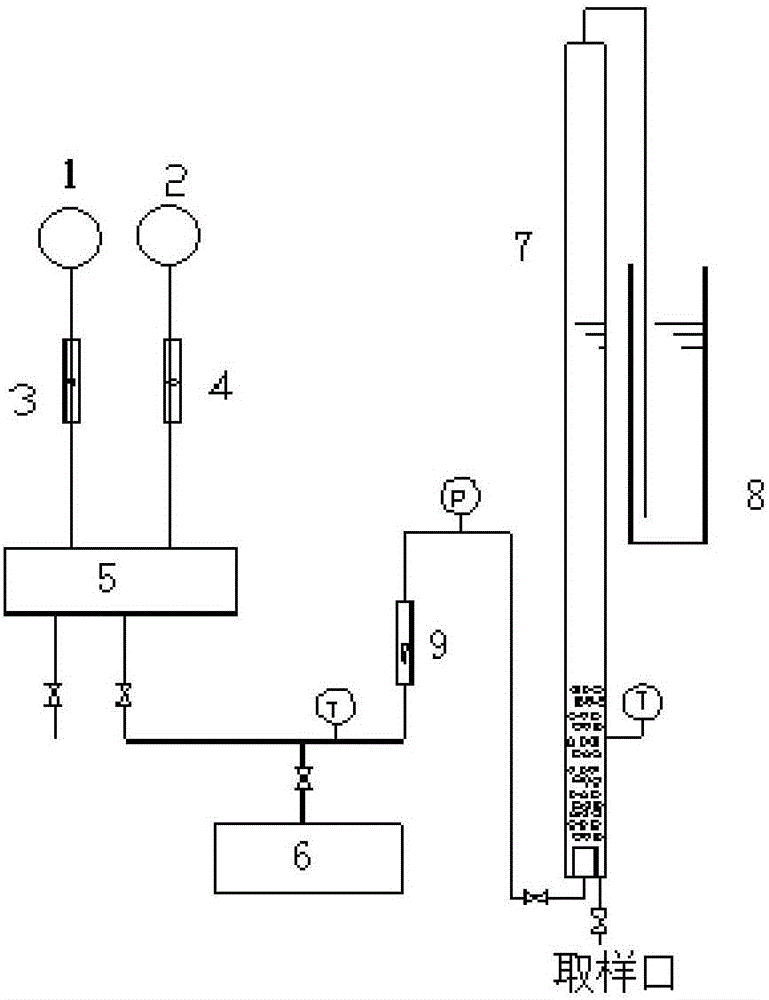
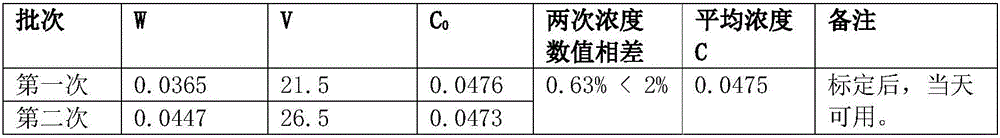
InactiveCN1865993APrevent liquefactionGood measurement precisionMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationIodometric methodPhysical chemistry
The disclosed combined detection method for high-pure SO3 gas with SO2 as impurity comprises: constant-volume sampling at least 60Deg better 60-80Deg to avoid liquidation of SO3, absorbing with alkali solution to obtain total volume percentage concentration of high-pure SO3 gas and SO2; processing the absorbed liquid, using iodometric method to obtain SO2 volume percentage concentration and then the SO3 concentration. This invention brings an error less than 0.1%.
Owner:SHANGHAI WUJING CHEM
One-step method for detecting concentration of chlorine and chlorinated hydrogen in pollution source waste gas
The invention discloses a one-step method for detecting the concentration of chlorine and chlorinated hydrogen in pollution source waste gas, which has the following steps of: connecting in series two absorption bottles respectively filled with 50 ml of alkali absorption solution with concentration of 0.1-0.2 mol / L for sampling; after collecting the samples, mixing sample solutions in the two bottles, and adding the alkali absorption solution with concentration of 0.1-0.2 mol / L until the total sample solution volume reaches 125 ml; moving 25 ml of solution from the total sample solution to an iodine flask for detecting the concentration of chlorine by using iodometry, and then moving 25 ml of solution sample solution for detecting the concentration of total chlorinated hydrogen in the sample solution by using silver nitrate volumetric method; and finally, calculating the actual concentration of chlorinated hydrogen by using a corrector formula. The method can be used for simultaneously analyzing chlorine and chlorinated hydrogen by one share of absorption solution, and eliminate the positive interference of chlorine to chlorinated hydrogen, thereby accurately determining the concentration of chlorine and chlorinated hydrogen in pollution source waste gas.
Owner:BAIYIN NONFERROUS GROUP
Test method for evaluating reaction proportion of nascent state oxygen during determination of ozone in water with iodometric method
Owner:CNOOC TIANJIN CHEM RES & DESIGN INST +1
Detection technology for basic copper chloride content
InactiveCN101464416AMaterial analysis by observing effect on chemical indicatorIodometric methodFiltration
The invention provides a technique for detecting the content of copper chloride hydroxide. Only copper chloride in the copper chloride hydroxide is capable of dissolving in water, while cupric hydroxide and copper chloride hydroxide are capable of dissolving in acid. The technique comprises the following steps: dissolving copper chloride hydroxide in water and then filtering, and measuring the content of water soluble chloride ion in the filtrate obtained from filtration by the Volhard titration method; dissolving copper chloride hydroxide in nitric acid, and measuring the total content of chloride ion therein by the Volhard titration method; and dissolving copper chloride hydroxide in hydrochloric acid, and measuring the total content of copper therein by the iodometric method. The accurate contents of water soluble copper chloride, copper chloride hydroxide and cupric hydroxide in copper chloride hydroxide can be calculated through the three steps.
Owner:SHANDONG NEW HOPE LIUHE GROUP
Determination method of furfuryl alcohol content in no-bake furan resin for casting
ActiveCN103105395AQuality assuranceEffective graspMaterial analysis by observing effect on chemical indicatorFuranIodometric method
The invention relates to a determination method of furfuryl alcohol content in no-bake furan resin for casting, and belongs to the technical field of detection. The determination method of furfuryl alcohol content in no-bake furan resin for casting comprises the following steps of: determining the content of main ingredient furfuryl alcohol in furan resin by adopting back titration of iodometry, wherein the reaction flow mainly comprises the following substeps of: firstly carrying out bromination reaction on samples, bromine, glacial acetic acid and hydrochloric acid; then dropping in potassium iodide for replacement reaction after bromination; and finally dropping in insodium thiosulfate for reaction. Through the determination method provided by the invention, the furfuryl alcohol content in no-bake furan resin for casting can be determined, so that on one hand, the furfuryl alcohol content can be grasped effectively when the no-bake furan resin is in check-out, the quality for using no-bake furan resin is ensured, the cast quality is stabilized, and the cast rejection rate caused by casts is lowered greatly, and on the other hand, the quality of resin can be evaluated through sample test when new suppliers are developed, so that the test cycle is shortened, the hazard and loss of material object test failure are lowered, and meanwhile basis is provided for confirming the price of resin.
Owner:江苏皓一机械制造有限公司
Five-step iodometric method
InactiveCN105403661ASolve difficult procurementSolve the inconvenience of carryingChemical analysis using titrationIodometric methodChlorine dioxide
The invention discloses a five-step iodometric method. According to the invention, in the third step of a conventional five-step iodometric method, solution pH value is adjusted to be 12 or higher instead of adding high-purity nitrogen, so that chlorine dioxide in a sample to be detected is transformed into a chlorite and chlorate which are not reacted, separation of chlorine dioxide with chlorine gas is realized, it is not necessary to blow chlorine dioxide and chlorine gas away with high-purity nitrogen, and problems that nitrogen blowing time is controlled to be longer than 40min, treatment time is too long, nitrogen is not easily available, is not convenient to carry, and is dangerous are solved; and in the fourth step, solution pH value is adjusted to be 3 to 7.
Owner:SHENZHEN SINSCHE TECH
Method for detecting copper content of ferric nitrate solution
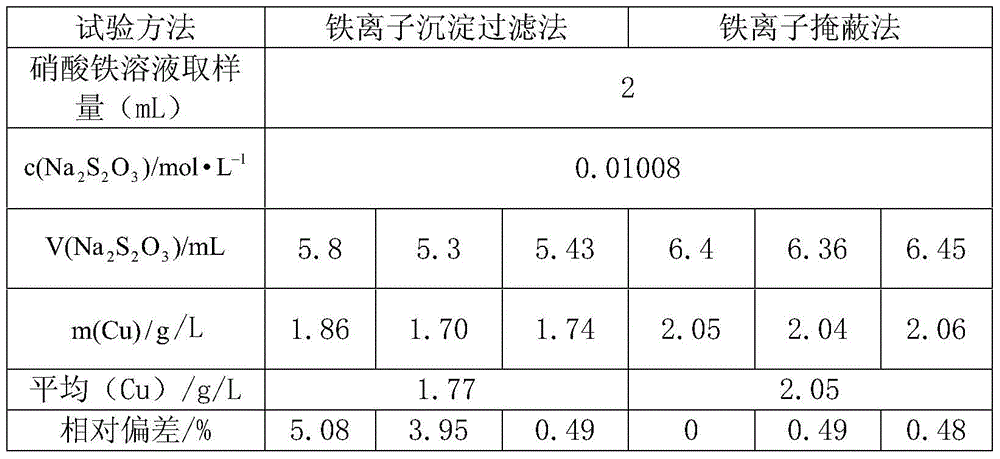
InactiveCN105699373AImprove accuracyShort analysis timeMaterial analysis by observing effect on chemical indicatorIodometric methodNitrate
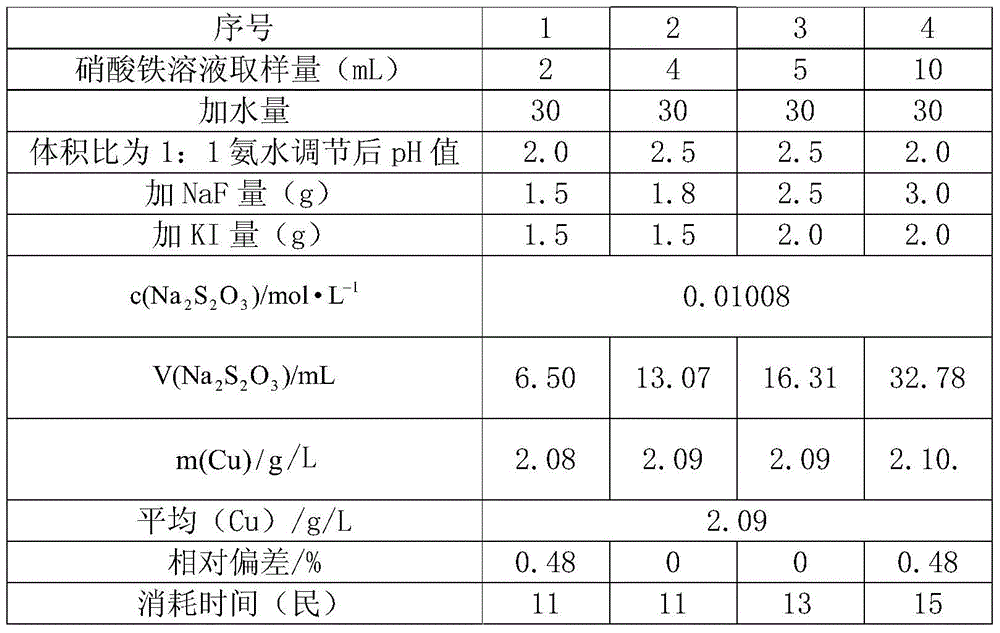
The invention relates to a method for detecting copper content of a ferric nitrate solution. By the adoption of the method for detecting copper ions in the ferric nitrate solution by an iodometric method, the time is greatly shortened, and the working efficiency is improved. The detection method comprises the following steps: (1) accurately transferring the ferric nitrate solution into a conical flask of 250 mL, and adding water of 30 to 50 mL; (2) titrating ammonia water with a volume ratio of 1: 1 to adjust the pH value of the ferric nitrate solution to 2.5; (3) adding a HAc-NaAc solution of 10 mL with the pH value of 3.6; (4) adding fluoride of 1.5 to 2.0 g, shaking the solution to discolor the solution from red to being colorless, wherein the fluoride is NaF; (5) adding a KI solution of 2.0 to 2.5 g, which is 2 to 3 times greater than the detection amount, to discolor the solution into dark yellow; and (6) titrating the solution to shallow yellow with a standard Na2S2O3 solution, adding a starch indicator solution of 3 mL to titrate the solution to shallow blue, and adding a NaSCN solution of 2mL to continuously titrate the solution until the blue disappears.
Owner:YANCON YULIN FINE CHEM CO LTD
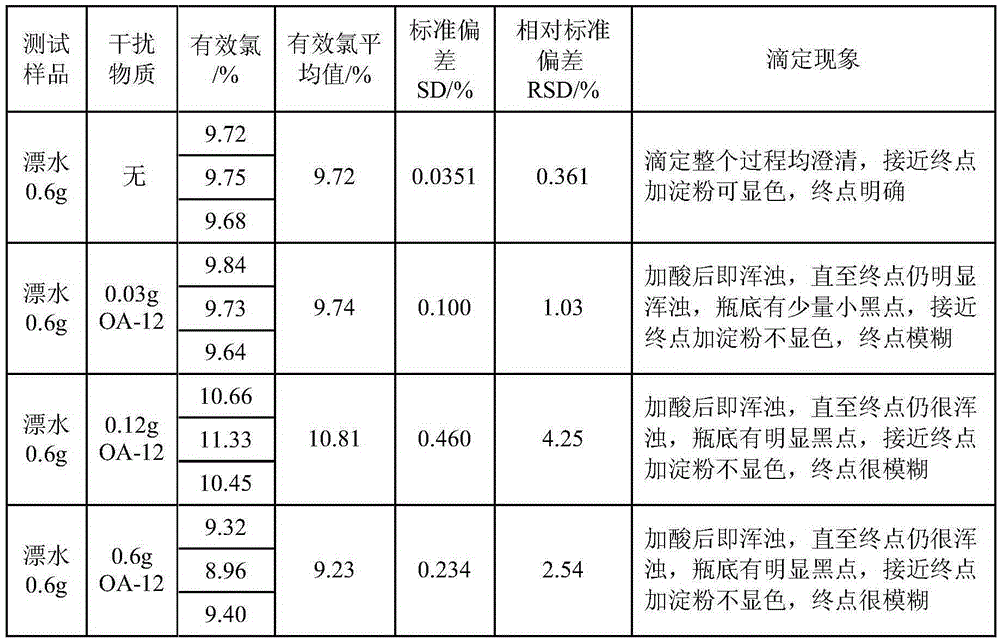
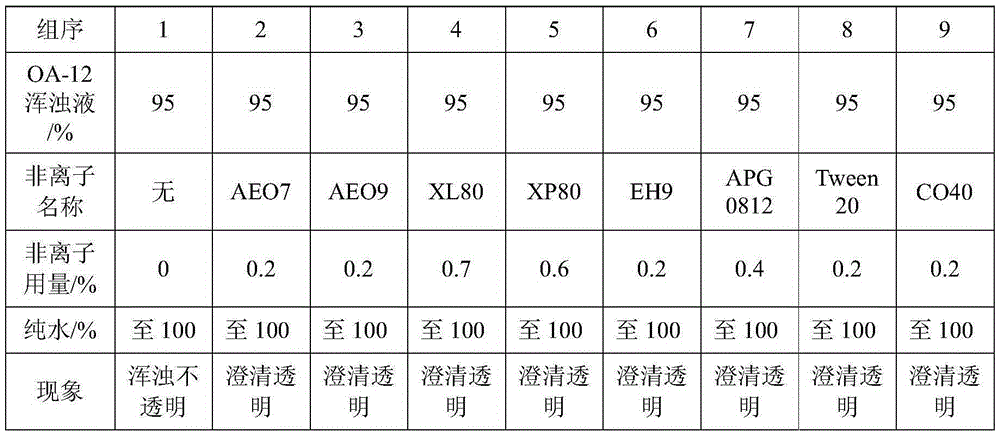
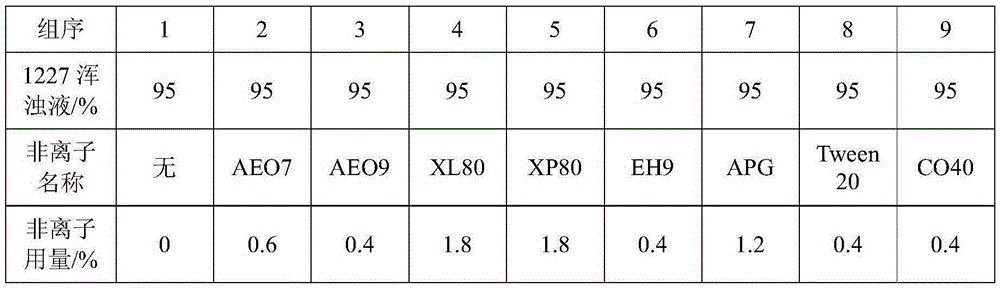
Method for improving iodometry measurement accuracy of samples containing interfering substances
ActiveCN105259300AOvercome the problem of inaccurate measurementReduce dosageChemical analysis using titrationIodometric methodIodine
The invention discloses a method for improving iodometry measurement accuracy of samples containing interfering substances. According to the method, a proper amount of nonionic surfactant is added on the basis of a conventional iodometry, and the problems that certain samples containing the interfering substances become turbid in the titration testing process and even accurate measurement is difficult due to iodine precipitation are successfully solved. By means of the method, a titration reaction is quick and sufficient, a titration end point is clear and transparent, the end point is accurately judged through color changes of iodine even if starch can not normally display colors, and a test result is good in precision and accuracy. Reagents used in the method are all conventional, safe, free of harm, easy to operate and suitable for being popularized in detection organizations and research institutions.
Owner:广州超威生物科技有限公司
Method for determining sulfur dioxide in amygdalin contained traditional Chinese medicine and slices thereof
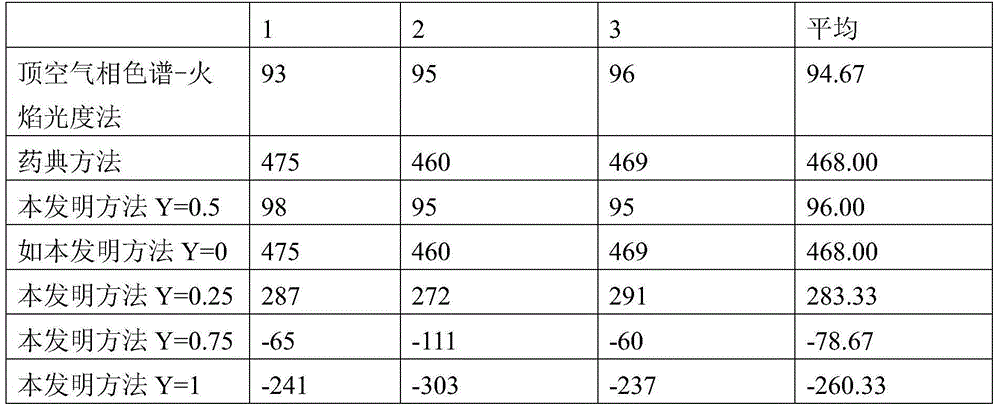
ActiveCN104865245AImprove accuracyHigh precisionMaterial analysis by observing effect on chemical indicatorComponent separationIodometric methodAmygdalin
The invention discloses a method for determining sulfur dioxide in amygdalin contained traditional Chinese medicine and slices thereof. An interference value of an iodometric method is eliminated due to the combination of the iodometric method and a silver nitrate titration method and namely that a difference value of the sulfur dioxide content which is determined by a titration method and the sulfur dioxide content which is determined through headspace gas chromatography and flame photometry is reduced to be within 2 milligrams every kilogram from 370 milligrams every kilogram. The method is simple and convenient, rapid and accurate.
Owner:四川省中药饮片有限责任公司
Iodometric method for determining oxygen vacancy concentration in barium ferrite-based lead-free piezoelectric ceramics
ActiveCN109115940AEasy to judgeAvoid measurement errorsChemical analysis using titrationOxygen vacancyComposition analysis
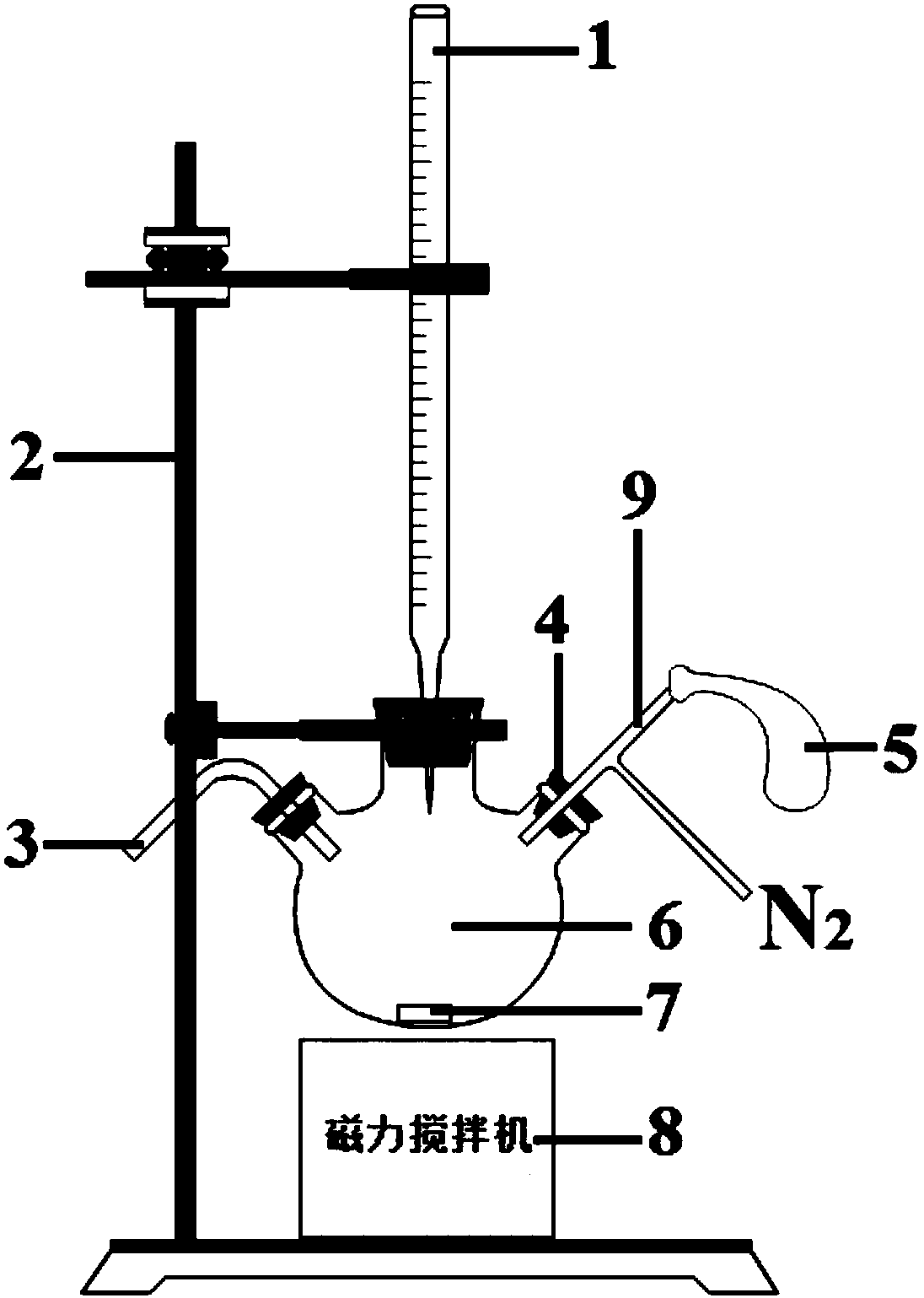
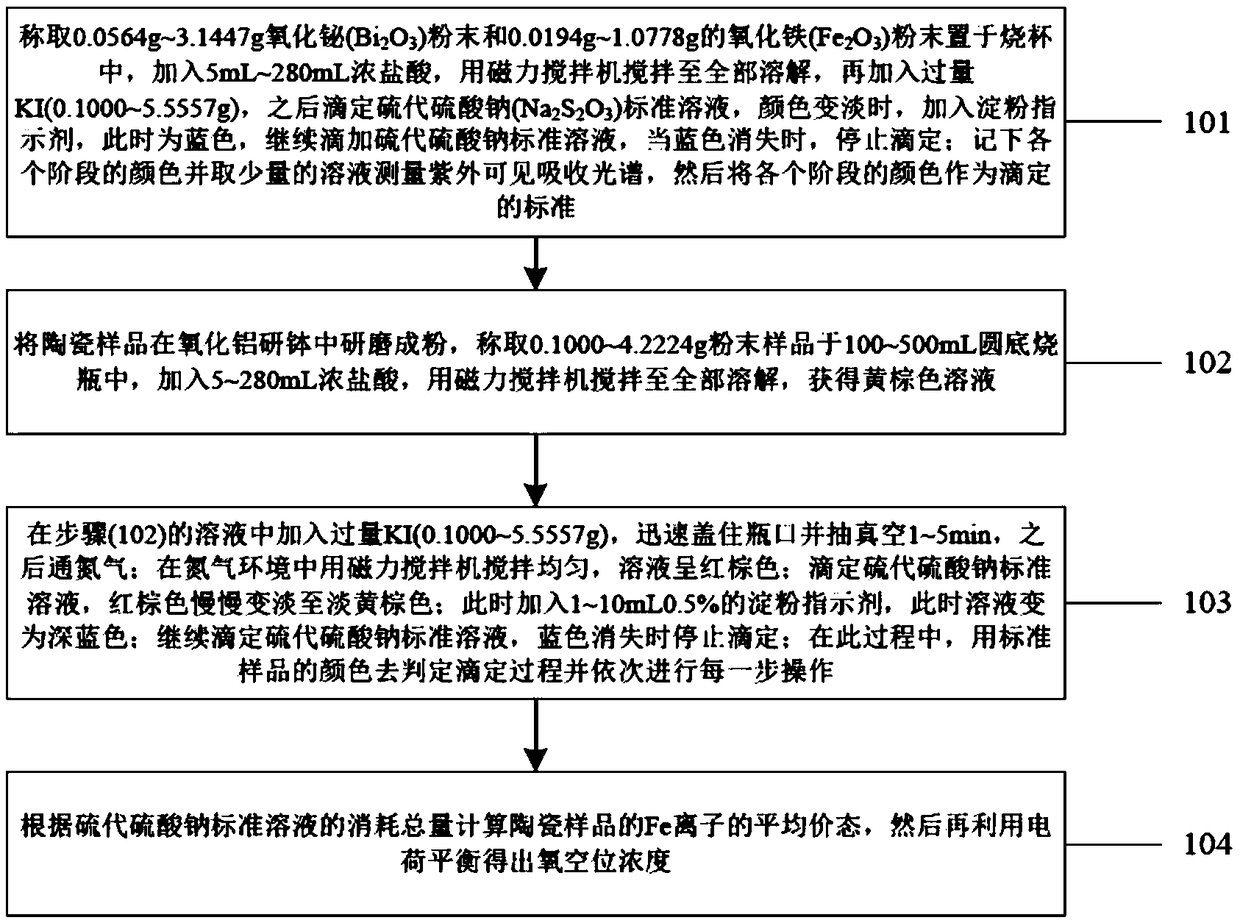
The invention discloses an iodometric method for determining oxygen vacancy concentration in barium ferrite-based lead-free piezoelectric ceramics, belonging to the technical field of ceramic composition analysis. The method comprises the following steps: taking a ceramic sample and grinding into a powder, weighing in a round-bottom flask; adding concentrated hydrochloric acid and stirring until the powder is completely dissolved, and marking the obtained solution in brown yellow color; adding a sodium thiosulfate standard solution into a burette, recording the number of the scale; adding potassium iodide into the brown yellow solution and covering with a plug; vacuuming and then introducing nitrogen gas, stirring evenly, and marking the obtained solution in reddish brown color; titratingto be light brown yellow with the sodium thiosulfate standard solution, adding a starch solution, and marking the obtained solution with dark blue; using the sodium thiosulfate standard solution to titrate until the blue color disappears; recording the numbers of the scales after the twice addition of the sodium thiosulfate standard solution, and calculating the total consumption amount of the sodium thiosulfate standard solution; calculating the valence state of Fe ions in the ceramic sample, and then utilizing charge balance to obtain the oxygen vacancy concentration. The iodometric method provided by the invention is simple and convenient in experimental instruments, simple to operate and accurate and reliable in detection results, and can realize the quantitative analysis of the oxygenvacancy concentration.
Owner:UNIV OF SCI & TECH BEIJING
Method for determining sulfur content in iron ore by combustion iodometry
ActiveCN102818876BThe measurement results are stable, accurate and reliableIncrease air velocityChemical analysis using combustionIodometric methodIronstone
The invention relates to a method for determining the sulfur content in iron ore by a combustion iodometry and belongs to the material test or analysis technology. According to the method, an iron ore test sample is placed in a porcelain boat and is put into a 1200-1300 DEG C tubular furnace to be heated for 1.52 minutes, a miniature air pump is started, exhausted air is introduced into the tubular furnace for sufficient burning, the burnt mixed gas is introduced into starch absorption liquid, the blue color of the starch absorption liquid starts to fade away, then, potassium iodate standard solution is immediately used for dripping into the starch absorption liquid, the blue color is recovered, when the color fading of the starch absorption liquid is slow, the dripping speed of the otassium iodate standard solution is decelerated until the color of the starch absorption liquid is same as the original blue color and is not changed, the miniature air pump is closed, the porcelain boat is taken out, the milliliter number of the dripped otassium iodate standard solution is read, and the sulfur content is calculated according to a formula S percent=T(V-V0) / W*100percent. The method has the advantages that the determined result of the sulfur content of the ore test sample is accurate and stable, the measurement process is safe, and no potential safety hazard exists.
Owner:WUHU XINXING DUCTILE IRON PIPES
Method for measuring content of antimony in methyltin mercaptide composite heat stabilizer through indirect iodometric method
InactiveCN106124690ADetect interferenceReliable pretreatmentChemical analysis using titrationPotassium iodineAntimony
The invention relates to a method for measuring content of antimony in a methyltin mercaptide composite heat stabilizer through an indirect iodometric method. The method comprises the following steps that firstly, a certain amount of methyltin mercaptide composite heat stabilizer samples are weighed, concentrated sulfuric acid, concentrated nitric acid and perchloric acid are sequentially added, the volume proportion of the concentrated sulfuric acid to the concentrated nitric acid to the perchloric acid is (2-3):1:1, the proportion of the sample mass to the mixed acid volume is 1: (35-55) g / ml, heating and dissolving are carried out, after the mixture is cooled to the room temperature, a (1+9) hydrochloric acid solution is added for constant volume V, and the mixture is shaken evenly to be used; secondly, the volume V0 of the solution prepared in the first step is moved and taken, a proper amount of potassium iodide and starch indicator are added, a sodium thiosulfate standard titration solution with the molar concentration being 1.000 mol / L is used for titration until the solution becomes colorless from blue so that a titration terminal can be achieved, the volume V1 of the consumed sodium thiosulfate standard titration solution is recorded, meanwhile, a blank test is made, volume V2 is recorded, and the antimony content is calculated according to formula 1 shown in the description. By means of the method, testing results are accurate and reliable, expensive special analytical instruments are not needed, the cost is low, and the method is easy to apply and popularize.
Owner:ZHEJIANG HIMPTON NEW MATERIAL
Method for identifying suckling pig liver and adult pig liver by utilizing catalase activity
InactiveCN113447607AReduce experimental errorChemical analysis using titrationMicrobiological testing/measurementBiotechnologyPig liver
The invention belongs to the field of enzyme activity detection, and relates to a method for identifying suckling pig liver and adult pig liver by utilizing catalase activity. The catalase is extracted from the pork liver by using an extraction method, the activity of the catalase in the pork liver is determined by using an iodometric method and a potassium permanganate method, and the detection method for identifying the adult pork liver and the suckling pork liver, which is high in accuracy, simple and convenient to operate and high in repeatability, is provided. Experiments show that under the same condition, the catalase activity of the suckling pig liver is obviously smaller than that of the adult pig liver, so that the catalase content in the pig liver can be used as an index for identifying the adult pig liver and the suckling pig liver; the method can fill the blank of methods and standards for detecting adult pig liver and suckling pig liver in China, provides a method which is high in accuracy, simple and convenient to operate and high in repeatability for detection of medicinal suckling pig liver raw materials, and has very high application value.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA ZHONGSHAN INST +1
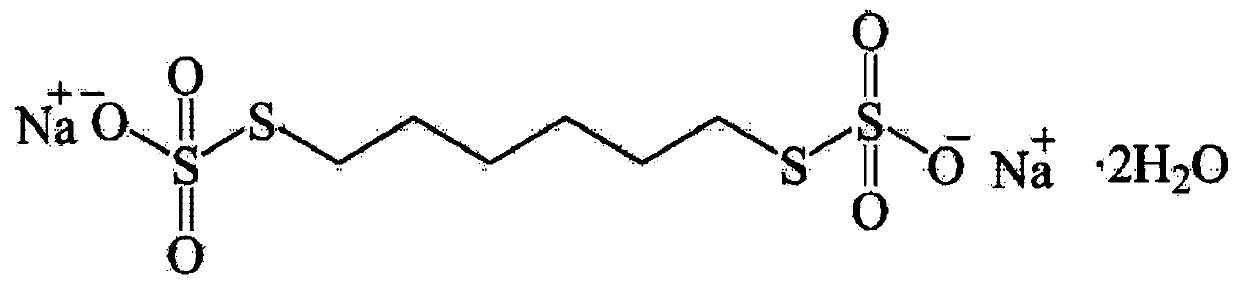
Method for detecting content of sodium thiosulfate in sodium hexamethylene-1,6-bisthiosulfate dehydrate
InactiveCN110208456AAvoid influenceEasy to operateChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorIodometric methodChloride
The invention provides a method for detecting the content of sodium thiosulfate in the sodium hexamethylene-1,6-bisthiosulfate dehydrate. A chloride ion content of a to-be-detected HTS sample is measured to avoid the influence on a detection result by chloride ions. When the to-be-detected sample is preprocessed, excessive silver nitrate solution and dilute nitric acid are added; and the dilute nitric acid decomposes sodium thiosulfate in the sample and thus the silver nitrate only reacts with the chloride ion in the HTS. After interference component removing, the content of sodium thiosulfatein the sample can be accurately detected by a simple silver method. The method has advantages of simple preprocessing process, simple operation, and low cost; the interference component in the detection process of the iodometric method can be removed fully; the detection result is accurate; and the method is suitable for large-scale popularization and application.
Owner:SHANDONG YANGGU HUATAI CHEM
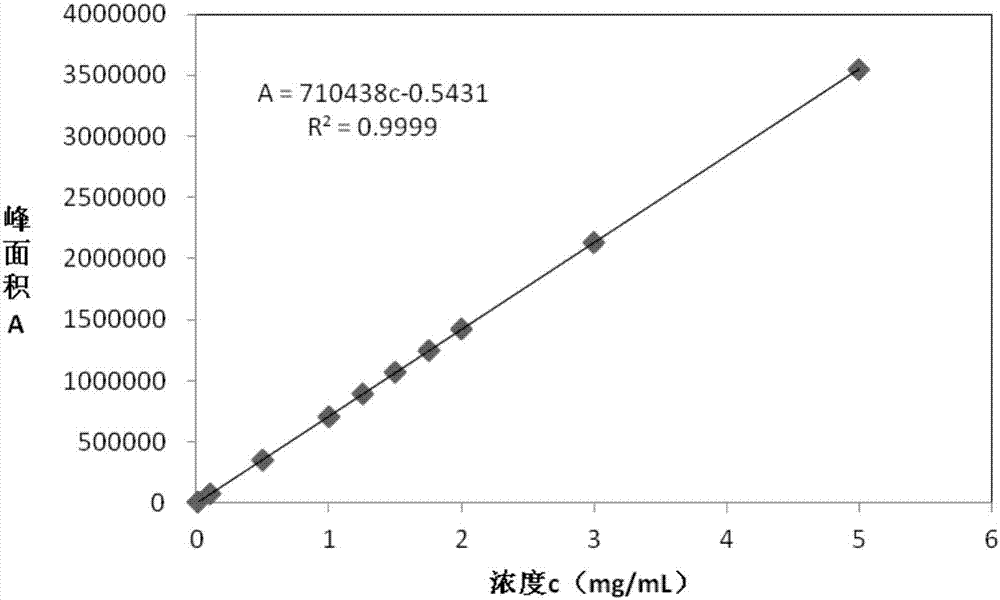
High performance liquid chromatography and application of sodium 3-(benzothiazol-2-ylthio)-propanesulfonate
ActiveCN106932514AHigh sensitivityMitigate imperfectionsComponent separationIodometric methodTest sample
The invention discloses a high performance liquid chromatography method and application of sodium 3-(benzothiazol-2-ylthio)-propanesulfonate. The method comprises the following steps: (a) providing a 3-(benzothiazol-2-ylthio)-propanesulfonate (ZPS) reference substance solution and a test sample solution; (b) analyzing the ZPS content of the test sample solution in step (a) with HPLC-UV according to an external standard method. Detection conditions are as follows: a chromatographic column uses octadecyl silane bonded silica gel as a filler; a mobile phase is formed by an organic phase and a phosphate buffer, the pH of a phosphate buffer is equal to 5.5 plus / minus 0.1, and the volume ratio of the organic phase to the phosphate buffer is (10 to 90) to (80 to 20); the detection wavelength is between 190nm and 330nm. The technical problems of imperfect measurement method of ZPS content, poor selectivity of iodometric method and poor accuracy are alleviated. The method can accurately detect the ZPS content, so that the precision is good, and the accuracy is high.
Owner:湖北吉和昌化工科技有限公司
Combined detection method for high-purity SO3 gas and impurity SO2 gas therein
InactiveCN100587487CPrevent liquefactionGood measurement precisionChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorIodometric methodPhysical chemistry
The disclosed combined detection method for high-pure SO3 gas with SO2 as impurity comprises: constant-volume sampling at least 60Deg better 60-80Deg to avoid liquidation of SO3, absorbing with alkalisolution to obtain total volume percentage concentration of high-pure SO3 gas and SO2; processing the absorbed liquid, using iodometric method to obtain SO2 volume percentage concentration and then the SO3 concentration. This invention brings an error less than 0.1%.
Owner:SHANGHAI WUJING CHEM
A method for determining sulfur dioxide in traditional Chinese medicine containing amygdalin and its decoction pieces
ActiveCN104865245BImprove accuracyHigh precisionComponent separationMaterial analysis by observing effect on chemical indicatorIodometric methodAmygdalin
The invention discloses a method for determining sulfur dioxide in amygdalin contained traditional Chinese medicine and slices thereof. An interference value of an iodometric method is eliminated due to the combination of the iodometric method and a silver nitrate titration method and namely that a difference value of the sulfur dioxide content which is determined by a titration method and the sulfur dioxide content which is determined through headspace gas chromatography and flame photometry is reduced to be within 2 milligrams every kilogram from 370 milligrams every kilogram. The method is simple and convenient, rapid and accurate.
Owner:四川省中药饮片有限责任公司
Catalyst chemical determination method for total hydrogen sulfide content in liquid sulfur
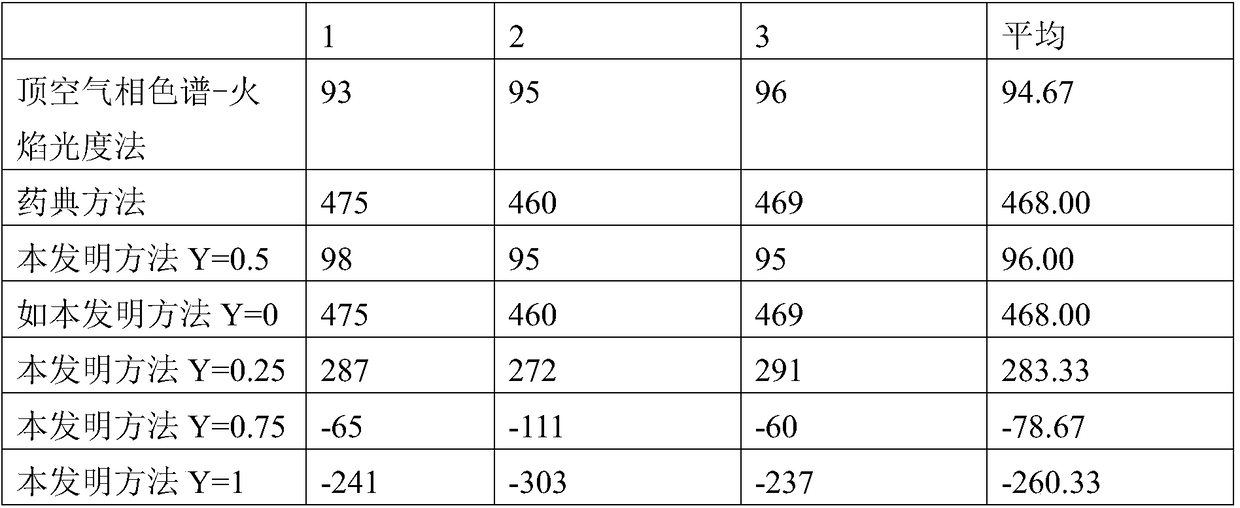
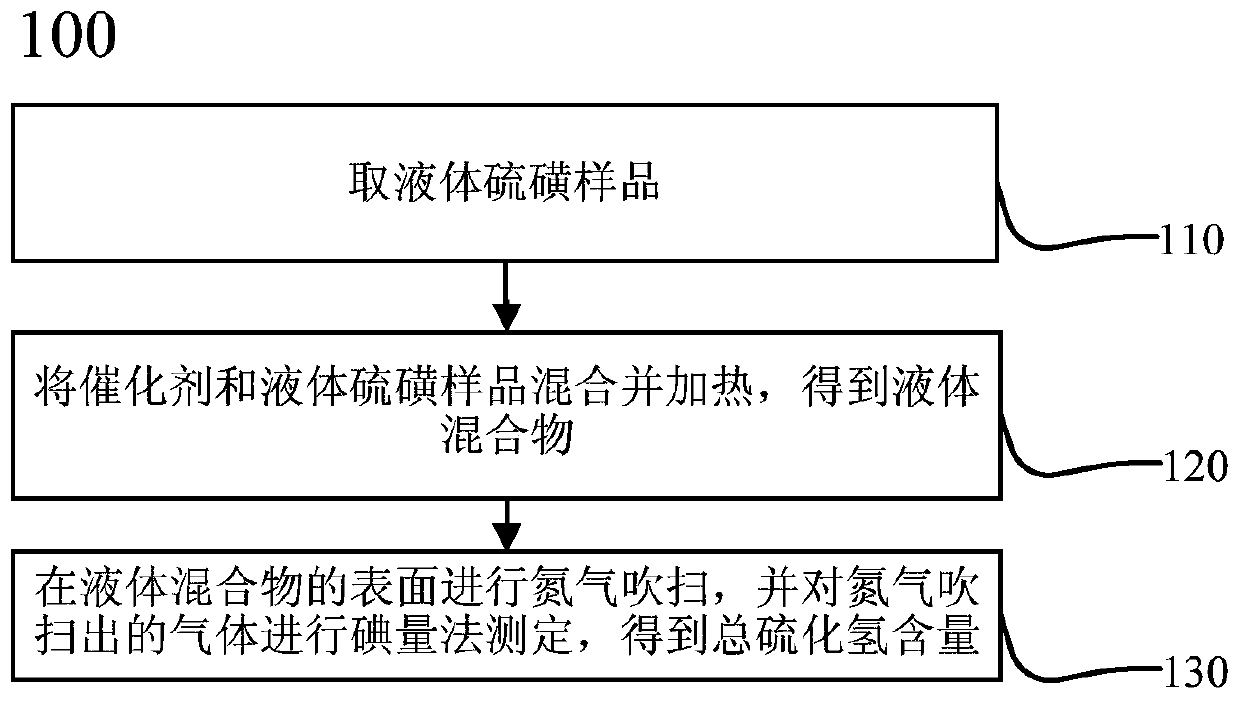
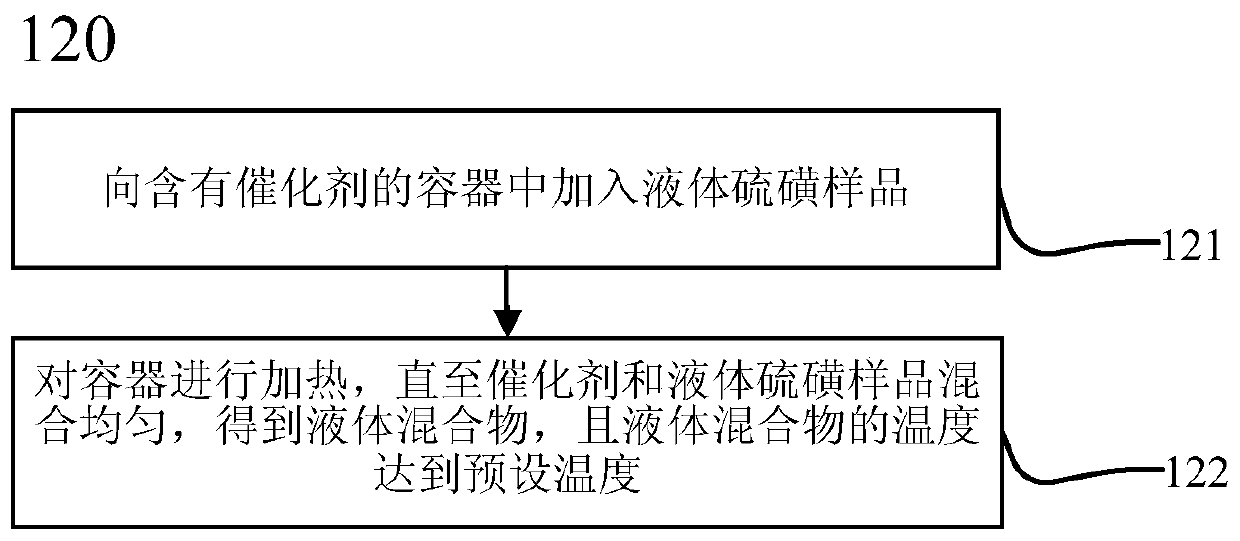
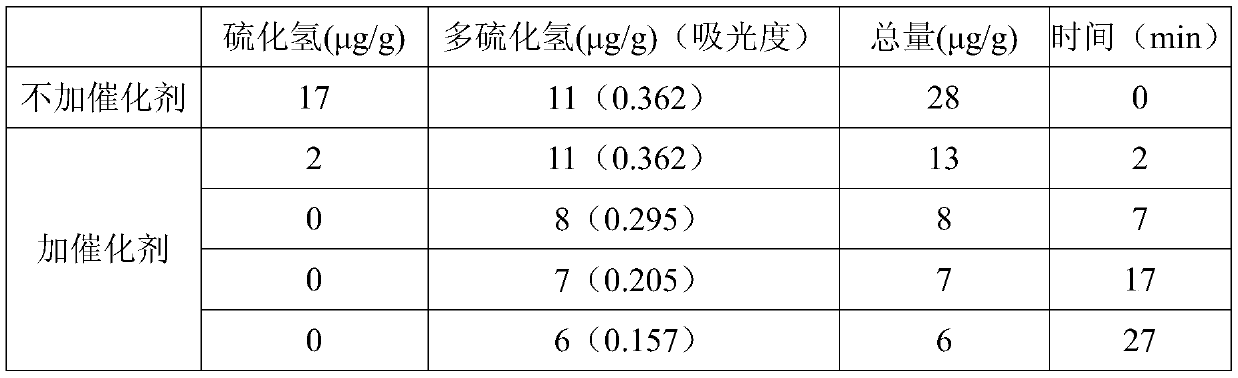
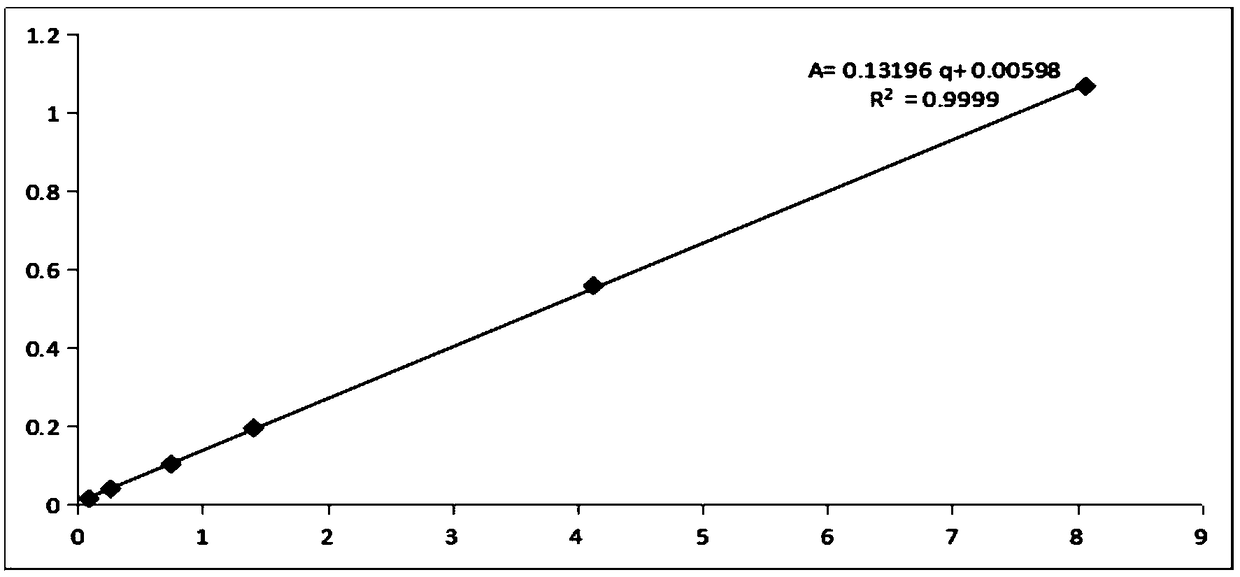
ActiveCN111474291AHigh measurement accuracyChemical analysis using catalysisIodometric methodPtru catalyst
The invention provides a catalyst chemical determination method for total hydrogen sulfide content in liquid sulfur. The method comprises the following steps: (1) mixing a catalyst and a liquid sulfursample, and heating to a preset temperature to obtain a liquid mixture; the catalyst comprising picric acid; (2) performing nitrogen purging on the surface of a liquid mixture, performing iodometry determination on gas obtained through nitrogen purging, and obtaining the total hydrogen sulfide content in liquid sulfur. The method comprises the following steps: adding a catalyst into a liquid sulfur sample and uniformly mixing; then, under the catalytic action of a catalyst, the equilibrium reaction in the liquid sulfur sample being broken; moving in the direction of generating hydrogen sulfide and hydrogen polysulfide; hydrogen sulfide and hydrogen polysulfide continuously overflowing from the surface of the liquid sulfur sample through nitrogen purging, so that the total hydrogen sulfidecontent in the liquid sulfur sample can be obtained. Experimental devices used in the method are simple, the operation is convenient and rapid, and the total hydrogen sulfide content in the liquid sulfur sample can be effectively and accurately measured.
Owner:PETROCHINA CO LTD
Method for determining content of tert-butyl peroxide in diesel oil by infrared spectroscopy
InactiveCN109358021AOvercoming time-consuming and solvent-consumingEasy to measureMaterial analysis by optical meansIodometric methodDecomposition
The invention provides a method for determining the content of tert-butyl peroxide in diesel oil by infrared spectroscopy. The invention overcomes the defects that an iodometric method is time-consuming and wastes solvents; the determination of tert-butyl peroxide by gas chromatography requires harsh temperature conditions to prevent decomposition; and a low temperature causes the diesel to fail to vaporize thereby contaminating an inlet, a column, and a detector, and resulting in poor reproducibility of the tert-butyl peroxide content determination. The method can conveniently accurately determine the content of tert-butyl peroxide in diesel oil.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
Method for detecting iron ions in hydrolysate prepared from buffalo horn powder and application of method in Qingkailing hydrolysate
ActiveCN113406069ASolve problems that are hard to measure accuratelyEliminate distractionsMaterial analysis by observing effect on chemical indicatorChemical analysis using titrationIodometric methodHydrolysate
The invention discloses a method for detecting iron ions in hydrolysate prepared from buffalo horn powder and application of the method in Qingkailing hydrolysate. According to the method disclosed by the invention, a to-be-detectedhydrolysate is pretreated, and an iodinated compound containing acetyl is added, so that the problem that the iron ions contained in the hydrolysate are difficult to accurately detect due to an end point color reversion phenomenon when the iron ions contained in the hydrolysate are detected by using the existing indirect iodometric method is solved, the interference of the background color of the hydrolysate is eliminated, the end point change is quickly judged through visual and clear color fading, the accuracy and stability of the detection result are guaranteed, real-time detection in industrial production can be realized, the production efficiency is greatly improved, and the product quality is guaranteed.
Owner:GUANGZHOU BAIYUNSHAN MINGXING PHARM CO LTD
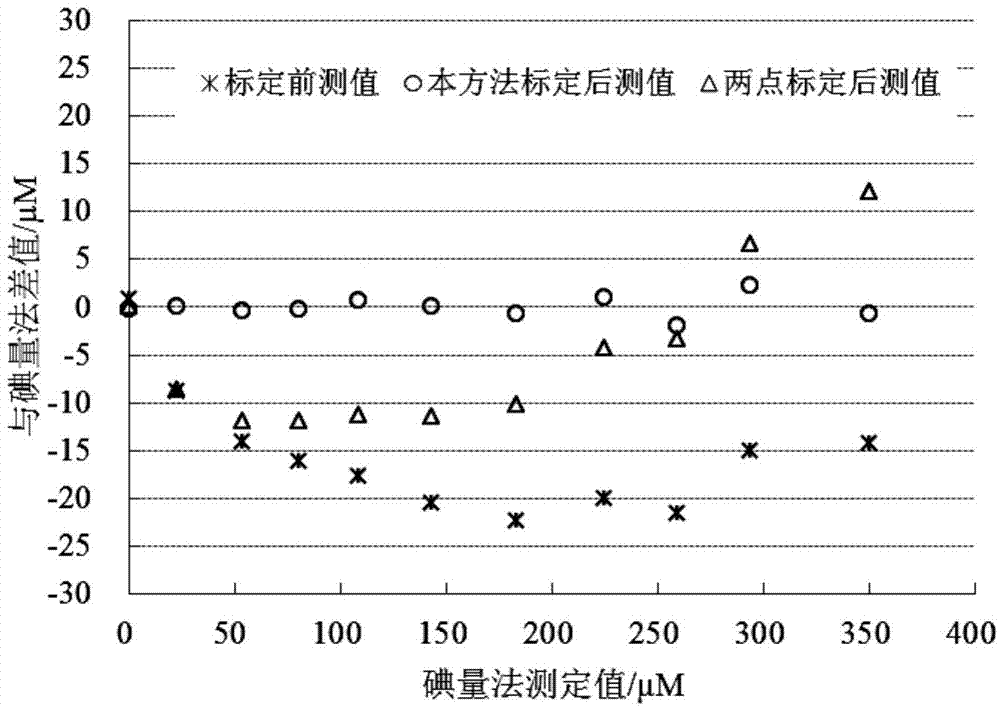
High-precision calibration method and device for optical dissolved oxygen sensor
ActiveCN104515761BFlexible settingsBaseline Accuracy ImprovementFluorescence/phosphorescenceIodometric methodSoil science
The invention relates to the technical field of water dissolved gas sensor calibration, and aims at providing a high-precision optical dissolved oxygen sensor calibration method and device. The high-precision optical dissolved oxygen sensor calibration method comprises the following steps: sequentially introducing mixed gases with different oxygen contents into a water-gas mixer, so as to supply multiple dissolved oxygen concentration gradients to a water body; recording a signal value of a dissolved oxygen sensor to be calibrated and a temperature value of the water body, and testing a standard dissolved oxygen value of a water sample by using an iodometric method; calculating a calibration coefficient of the dissolved oxygen sensor to be calibrated. As a practical iodometric method measured value obtained after synchronous sampling is taken as the standard value, the reference value precision of the method is greatly improved when being compared with conventional methods that the concentration of dissolved oxygen in water bodies is calculated according to the water dissolution degree of the mixed gas oxygen concentration; as five temperatures are adopted for sensor calibration, the calibration result is relatively wide in temperature application range when being compared with those of the conventional methods.
Owner:SECOND INST OF OCEANOGRAPHY MNR
High performance liquid chromatographic analysis method and application of 3-(benzothiazole-2-mercapto)-propanesulfonate sodium
Owner:湖北吉和昌化工科技有限公司
Method for detecting content of thiourea in hydrogen sulfide solution
ActiveCN102967601BAccurate measurementImprove accuracyMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationIodometric methodThiourea
The invention relates to a method for detecting content of a certain component in a mixture, and in particular relates to a method for detecting the content of thiourea in a hydrogen sulfide solution. The content of the thiourea in the hydrogen sulfide solution is detected by iodometry, and pretreatment is performed on the solution before the thiourea content is measured by the iodometry, wherein the pretreatment comprises the step of adding metal salt solution into the hydrogen sulfide solution to remove sulfhydryl groups and negative bivalent sulfur ions in the hydrogen sulfide solution. The method is high in accuracy, low in relative standard deviation, high in precision, high in reproducibility, easy, convenient and rapid to operate, low in detection cost and wide in application range and can be used for accurately measuring the thiourea content in the hydrogen sulfide solution.
Owner:SHANDONG EFIRM BIOCHEMISTRY & ENVIRONMENTAL PROTECTION CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com