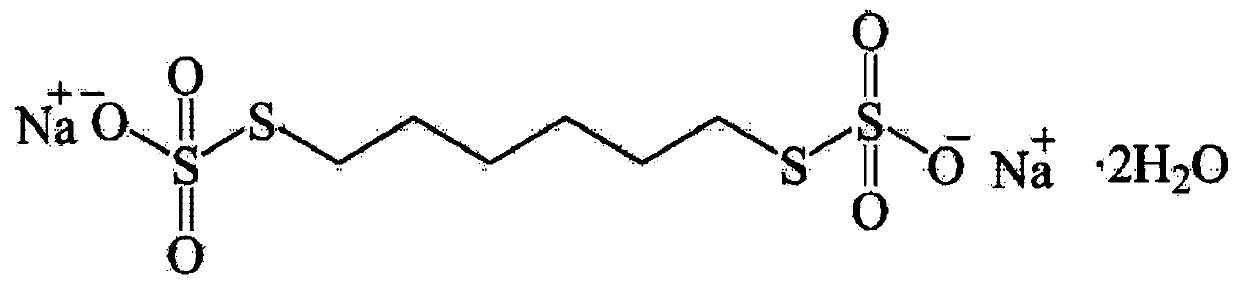
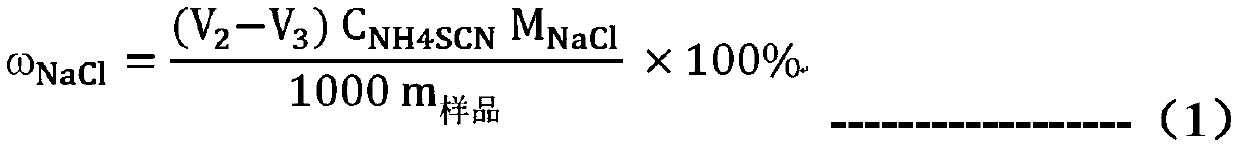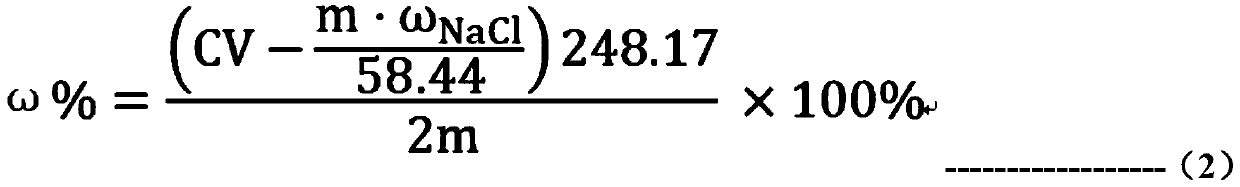Method for detecting content of sodium thiosulfate in sodium hexamethylene-1,6-bisthiosulfate dehydrate
A technology of disodium dithiosulfate and hexamethylene dihydrate, which is applied in the field of impurity detection, can solve problems affecting the accuracy of detection results, and achieve the effect of being suitable for large-scale application, accurate detection results, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The detection method of sodium thiosulfate content in the dihydrate hexamethylene-1,6-dithiosulfate disodium salt provided by the present invention is used to measure the sodium thiosulfate solution of known concentration (in this solution Add a certain quality of sodium chloride standard substance), to verify the detection method of the present invention, the steps are as follows:
[0049] Step 1: Solution configuration and calibration:
[0050] Ferric ammonium sulfate indicator: Dissolve about 5g of ferric ammonium sulfate dodecahydrate in 50ml of distilled water with a little nitric acid added dropwise.
[0051] Dilute nitric acid solution: Mix concentrated nitric acid and distilled water at a volume ratio of 1:3.
[0052] Preparation and calibration of silver nitrate solution:
[0053] Preparation: Accurately weigh 8.50g of silver nitrate, dissolve it in distilled water, and use a 500ml brown volumetric flask for constant volume to obtain a silver nitrate solution...
Embodiment 2
[0085] Determination of the content of sodium thiosulfate in unknown HTS products, the steps are as follows:
[0086] Step 1 is the same as the step in Example 1; prepare dilute nitric acid solution, 0.1mol / L silver nitrate solution, 0.025mol / L ammonium thiocyanate obtained by ferric ammonium sulfate indicator, concentrated nitric acid and distilled water with a volume ratio of 1:3 solution;
[0087] Step 2: Determination of chloride content (calculated as sodium chloride): add 0.2010g HTS sample and 30ml ultrapure aqueous solution in 100ml Erlenmeyer flask, first add 10ml dilute nitric acid solution (10%), add 5ml 0.1mol / L Silver nitrate solution (if there is a small amount of sodium thiosulfate remaining in the sample, the solution will turn orange-red or black precipitate will appear). Slowly heat the solution to be tested until the solution turns from cloudy to transparent and colorless, with a small amount of white precipitate at the bottom. After the solution is cooled...
Embodiment 3
[0103] Weigh 0.2217g of the HTS sample in Example 2 and mix it with a sodium thiosulfate solution with a known content of 0.004g as the sample to be tested. The relationship between the measured value and the theoretical value is compared, so as to further verify the accuracy of the test method of the present invention.
[0104] Step 1 is the same as the step in Example 1; prepare dilute nitric acid solution, 0.1mol / L silver nitrate solution, 0.025mol / L ammonium thiocyanate obtained by ferric ammonium sulfate indicator, concentrated nitric acid and distilled water with a volume ratio of 1:3 solution;
[0105] Step 2: Determination of chloride content (in sodium chloride): 0.2257g of the sample to be tested is dissolved in 10ml of water, first add 10ml of nitric acid solution (10%), add 5ml of 0.1mol / L silver nitrate solution (if in the sample There is a small amount of sodium thiosulfate remaining, the solution will turn orange-red or a black precipitate will appear). Slowly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average volume | aaaaa | aaaaa |
| Average volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com