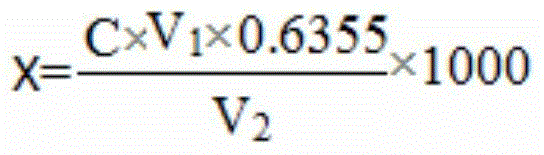Method for detecting copper content of ferric nitrate solution
A detection method, ferric nitrate technology, is applied in the direction of analyzing the material by chemical reaction and analyzing the material by observing the influence on the chemical indicator, which can solve the problems of time-consuming and low recovery rate of copper ions, and achieve saving The effect of working time, improving accuracy, and improving work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
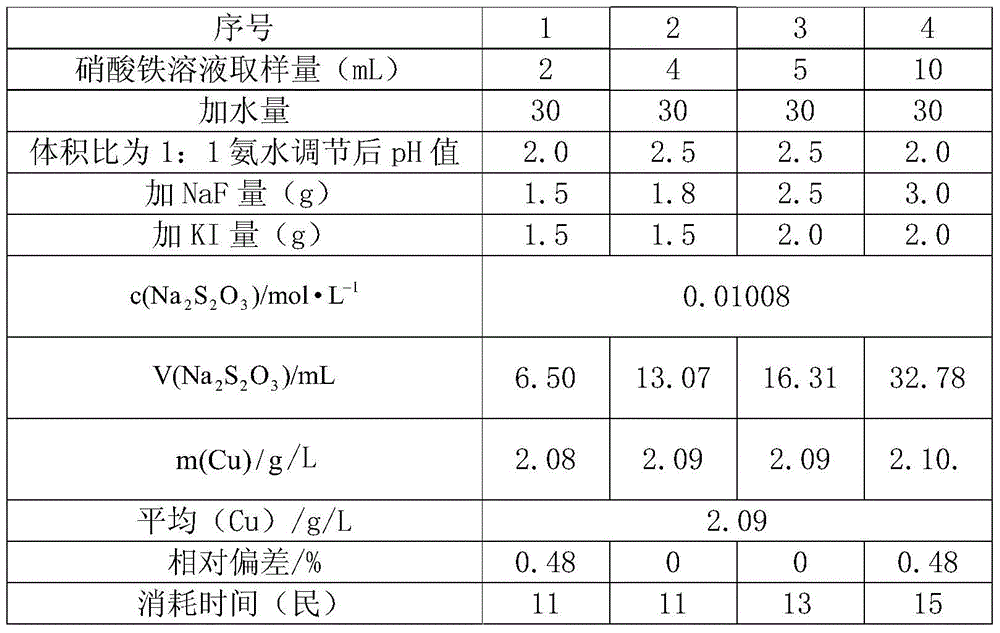
[0023] Embodiment: Accurately pipette 2ml, 4ml, 5ml, 10ml different amounts of ferric nitrate solution to carry out experimental detection according to the method steps of this method, and the specific results are shown in the table below.
[0024]
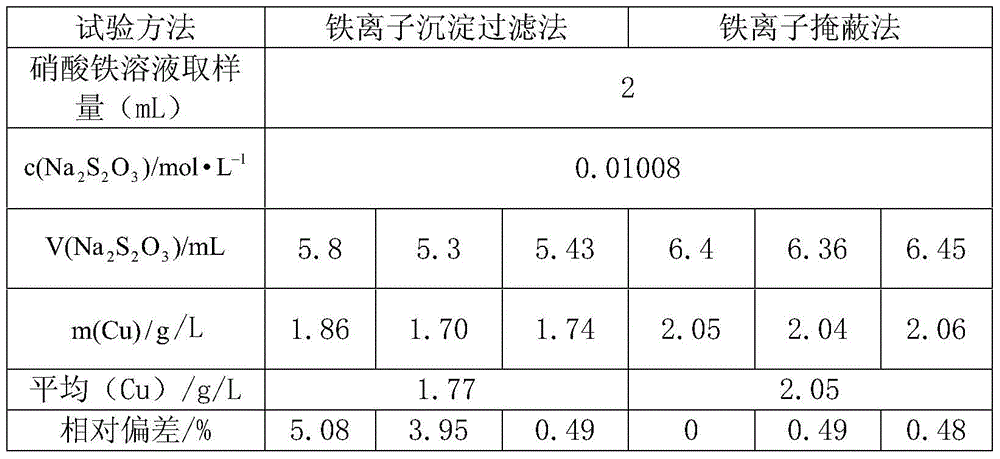
[0025] It can be seen from the above table that when pipetting 2ml, 4ml, 5ml, and 10ml of ferric nitrate solutions of different volumes, the method has good repeatability of the results of copper content detection in the solution, and the time consumed by the experiment can be controlled within 20 minutes. . Therefore, in the detection of the copper content in the ferric nitrate solution, the method can effectively improve the repeatability of the experiment, greatly shorten the experiment time, and fully demonstrate the simplicity and practicability of the method.
[0026] 1. Experimental principle of the present invention:
[0027] In weakly acidic solution (pH=3~4), Cu 2+ Interact with excess KI to generate CuI precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com