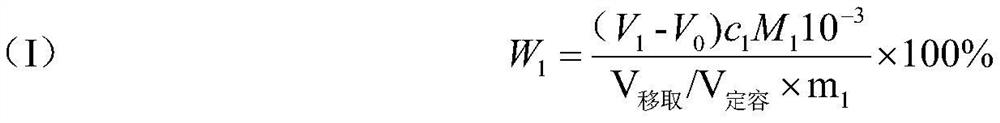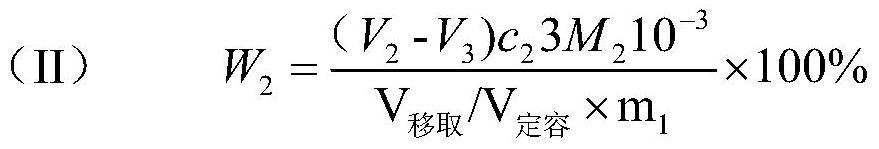Method for determining contents of fluosilicic acid, hydrofluoric acid and nitric acid in etching acid
A technology of nitric acid content and determination method, which is applied to electrochemical variables of materials, material analysis by observing the influence on chemical indicators, and analysis by chemical reaction of materials, etc., can solve the problem of low analysis cost, complicated pretreatment, The problem of high detection cost can achieve the effect of good result accuracy and repeatability, simple method operation and good method selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
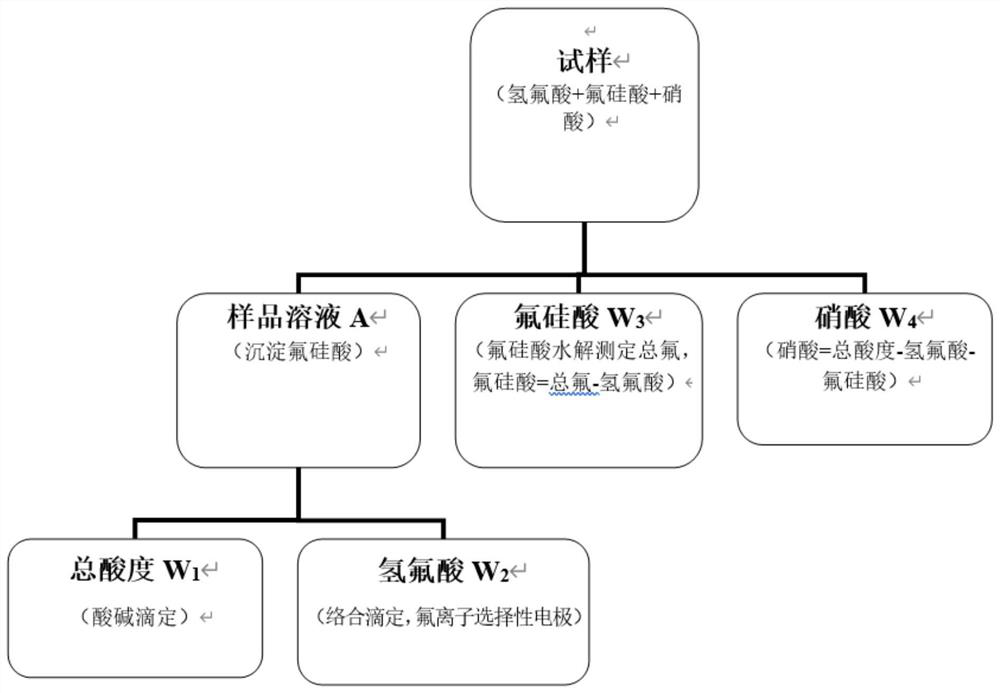
[0064] (1) Preparation of sample solution: Weigh about 2.4543g of the sample, put it in a 100mL plastic volumetric flask, add 10mL of saturated potassium nitrate solution, precipitate fluosilicic acid, add absolute ethanol and shake, and wait for the solution to cool to room temperature , use absolute ethanol to set the volume to the mark, let it stand until the precipitation layers, take the supernatant for the determination of total acidity, hydrofluoric acid and fluorosilicic acid, this is the sample solution A.
[0065] (2) Determination of total acidity: Add 30mL of water to a 250mL plastic beaker, pipette 25mL of solution A into the plastic beaker, add 3 drops of phenolphthalein indicator solution dropwise, and titrate with 0.5013mol / L sodium hydroxide standard titration solution until the solution is slightly The end point is pink and does not fade for 30s. The titration volume of the sample is 11.68mL, and the titration volume of the blank is 0.02mL. The total acidity ...
Embodiment 2
[0075] (1) Preparation of the sample solution: Weigh about 1.0065g of the sample, put it in a 100mL plastic volumetric flask, add 10mL of saturated potassium chloride solution, precipitate the fluorosilicic acid, add anhydrous methanol and shake, and wait for the solution to cool to room temperature Finally, use anhydrous methanol to make the volume up to the mark, let it stand until the precipitation layers, and take the supernatant for the determination of total acidity, hydrofluoric acid and fluorosilicic acid, which is sample solution A.
[0076] (2) Determination of total acidity: Add 30mL of water to a 250mL plastic beaker, pipette 25mL of solution A into the plastic beaker, add 3 drops of phenolphthalein indicator solution dropwise, and titrate with 0.5013mol / L sodium hydroxide standard titration solution until the solution is slightly The end point is pink and does not fade for 30s. The titration volume of the sample is 6.34mL, and the titration volume of the blank is 0...
Embodiment 3
[0080] Embodiment 3 accuracy verification
[0081] Weigh 99.66g of 34.82% hydrofluoric acid solution, 115.39g of 31.74% fluosilicic acid solution, 200.13g of 60.18% nitric acid and 50.09g of water to prepare a simulated sample. The theoretical values of the content of the three mixed acids in the simulated sample are: 7.46% hydrofluoric acid, 7.87% fluosilicic acid, and 25.89% nitric acid. Weigh 1.0522g, 1.3738g, 1.5660g, 1.9009g, 2.2177g, and 2.4990g to simulate sample, prepare 6 samples of solution A in parallel, measure the total acidity and hydrofluoric acid content; weigh 0.2737g, 0.3003g, 0.3760g, 0.4082g, 0.4661g, 0.5003g to determine the total fluorine content, and measure the solution hydrofluoric acid according to the determination steps acid, fluosilicic acid and nitric acid concentrations. The measurement results are as follows:
[0082]
[0083] The repeatability RSD of the determination results of the three acid contents is less than 2%, and the deviation fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com