A kind of assay method of sodium oleate content in dry emulsion for injection
A determination method and dry emulsion technology, which are applied in the field of determination of sodium oleate content in dry emulsion for injection, can solve problems such as difficulty in separation and detection, and achieve the effects of sensitive and low-cost determination methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 The establishment process of this method
[0032] Experimental materials, instruments and chromatographic conditions
[0033] Sodium oleate supplementary material was purchased from Xi'an Libang Pharmaceutical Co., Ltd., and the oleic acid content in COA was 82.8% (gas chromatography), and the oleic acid reference substance was purchased from China Food and Drug Control Research Institute, and the content was 99.6%.
[0034] High performance liquid chromatography Waters e2695, the detector is UV detector; the chromatographic column is ZORBAX SB C8, 4.6mm*150mm, 5μm; column temperature: 40℃; detection wavelength is 210nm; injection volume: 60μl; flow rate: 1.0ml / min; mobile phase: 0.02M potassium dihydrogen phosphate buffer (pH=2.0)-acetonitrile gradient elution; MPA: 0.02M potassium dihydrogen phosphate buffer (pH=2.0), MPB: acetonitrile, MPA-MPB gradient elution Take off:
[0035] Elution condition:
[0036]
[0037] Experimental procedure
[0038]...
Embodiment 2
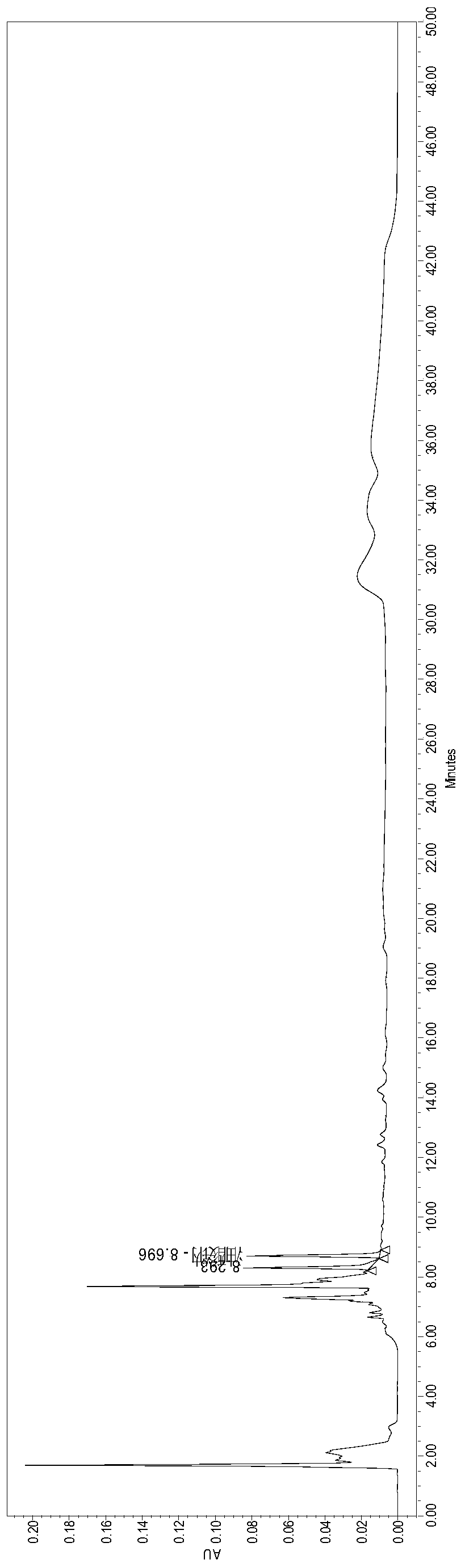
[0043] Experimental materials, instruments and chromatographic conditions
[0044] Alprostadil dry emulsion for injection, wherein the input amount of sodium oleate auxiliary material is 0.2mg / 250mg, sodium oleate auxiliary material is purchased from Xi'an Libang Pharmaceutical Co., Ltd., oleic acid reference substance is purchased from China Institute for Food and Drug Control, and the content is 99.6%.
[0045] Chromatographic condition is identical with embodiment 1
[0046] Experimental procedure
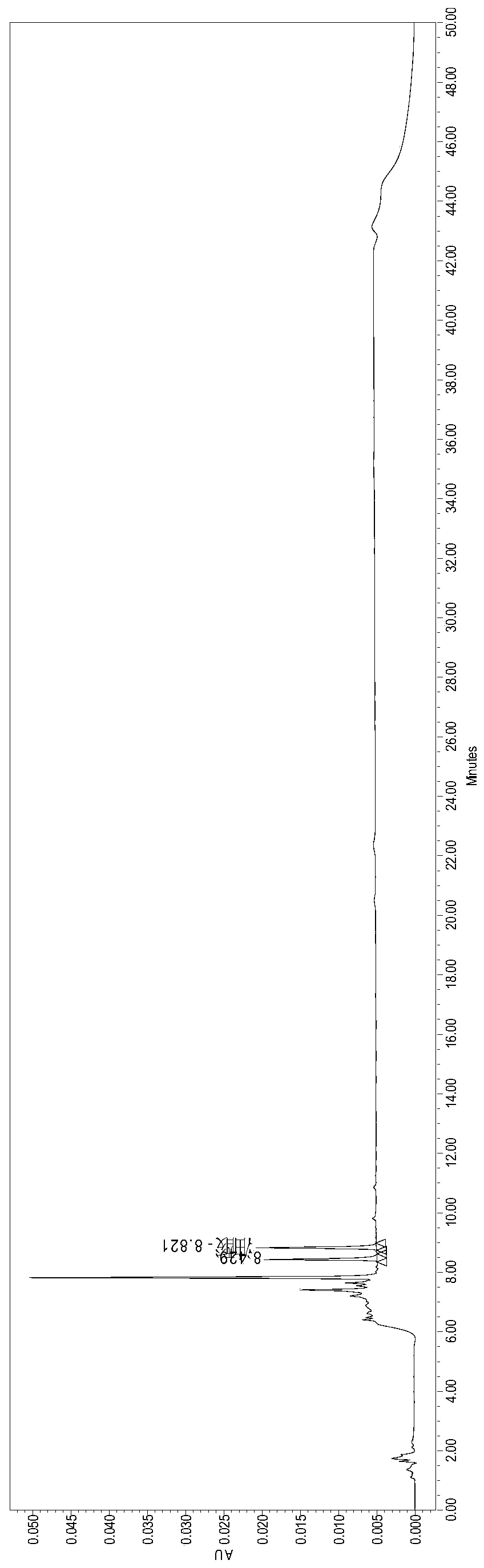
[0047] Take about 250mg of alprostadil dry emulsion powder for injection, accurately weigh it, put it in a volumetric flask, add 2.0ml of water, re-emulsion, add ethanol to make the volume to 5.0ml, shake for 2min, ultrasonic for 2.0min, and stand at room temperature After 30 minutes, an appropriate amount (2 mL) of the solution was centrifuged (15000 rpm, 15 minutes), and the supernatant was injected. Get an appropriate amount of oleic acid reference substance, dissolve and ...
Embodiment 3
[0050] Experimental materials, instruments and chromatographic conditions
[0051] Alprostadil blank dry emulsion for injection (without sodium oleate auxiliary material, other components are dosed according to the proportion of the formulation prescription), and the oleic acid reference substance was purchased from China National Institute for Food and Drug Control, with a content of 99.6%.
[0052] Chromatographic condition is identical with embodiment 1
[0053] Experimental procedure
[0054] Take about 250mg of the blank dry emulsion powder of alprostadil for injection, place it in a volumetric flask, add 2.0ml of water, re-emulsion, add ethanol to make the volume to 5.0ml, shake for 2min, ultrasonic for 2.0min, stand at room temperature Set aside for 30min, take an appropriate amount (2mL) of the solution and centrifuge (15000rpm, 15min), and take the supernatant to obtain the blank dry emulsion sample solution of alprostadil for injection.
[0055] Take an appropriate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com