Method for detecting content of telbivudine in blood plasma
A technology of telbivudine and content, which is applied in the field of medical testing, can solve the problems that the detection method and methodological verification have not been reported in detail, cannot meet the measurement requirements, and require a large amount of mobile phase, so as to avoid mutual interference and endogenous The interference of sexual substances, the simplified sample pretreatment method, and the effect of small sampling volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
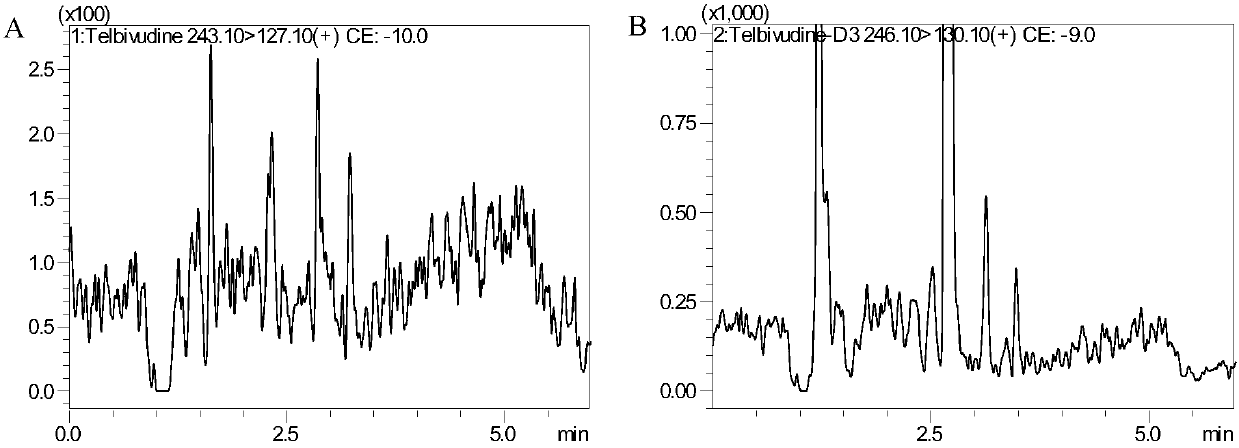
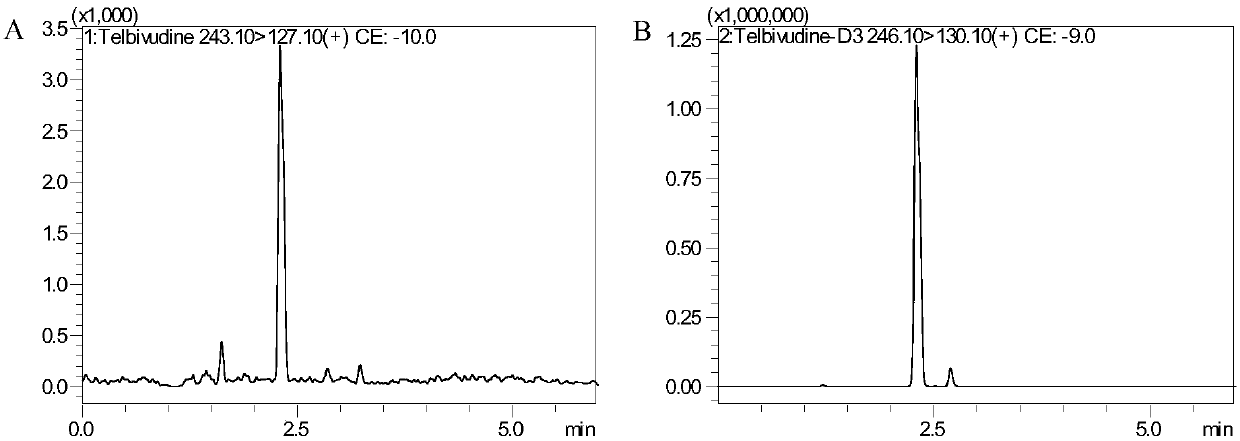
[0049] Chromatographic conditions
[0050] Japan Shimadzu LCMS8050 system: SIL-30AC autosampler (1 set), LC-30AD infusion pump (2 sets), DGU-20A 5 Online degasser (1 set), CBM-20A controller (1 set), CTO-30A column thermostat (1 set). Data acquisition and processing software: LabSolutions Ver.5.56SP1. Shimadzu Inertsil Sustain C18, 3.0×100mm, 3μm. Column temperature: 40°C. Mobile phase: 0.1% formic acid aqueous solution (A) and acetonitrile (B), gradient elution: 0-4.0min A:B from (95:5, v / v) to (20:80, v / v), 4.0- 4.1min A:B from (20:80,v / v) to (95:5,v / v), 4.1-6.0minA:B(95:5,v / v). Flow rate: 0.4mL / min.
[0051] Mass Spectrometry Conditions:
[0052] Japan Shimadzu company LCMS8050 mass spectrometer. Mass spectrometer ionization method: electrospray ion source; polarity: positive ion detection; scanning method: multiple reaction ion detection scanning (MRM); ion channel selection: LdT: m / z 243.10→127.10, D3-LdT: m / z 246.10→ 130.10; Collision gas: nitrogen.
[0053] The...
Embodiment 2
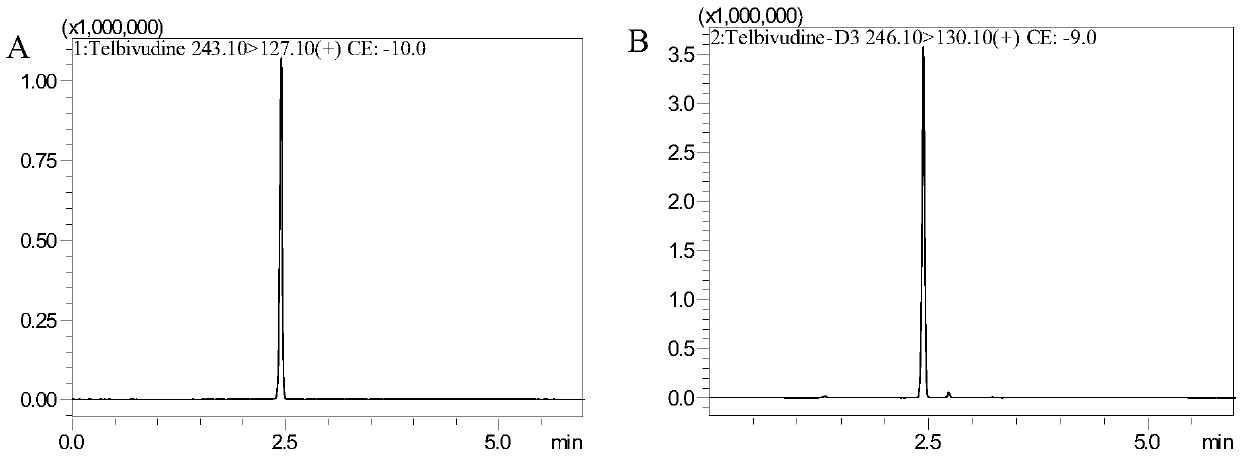
[0064] Chromatographic conditions
[0065] Japan Shimadzu LCMS8050 system: SIL-30AC autosampler (1 set), LC-30AD infusion pump (2 sets), DGU-20A 5 Online degasser (1 set), CBM-20A controller (1 set), CTO-30A column thermostat (1 set). Data acquisition and processing software: LabSolutions Ver.5.56SP1. Shimadzu Inertsil Sustain C18, 3.0×100mm, 3μm. Column temperature: 40°C. Mobile phase: 0.1% formic acid aqueous solution (A) and acetonitrile (B), gradient elution: 0-4.0min A:B from (95:5, v / v) to (20:80, v / v), 4.0- 4.1min A:B from (20:80,v / v) to (95:5,v / v), 4.1-6.0minA:B(95:5,v / v). Flow rate: 0.4mL / min.
[0066] Mass Spectrometry Conditions:
[0067] Japan Shimadzu company LCMS8050 mass spectrometer. Mass spectrometer ionization method: electrospray ion source; polarity: positive ion detection; scanning method: multiple reaction ion detection scanning (MRM); ion channel selection: LdT: m / z 243.10→127.10, D3-LdT: m / z 246.10→ 130.10; Collision gas: nitrogen.
[0068] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com