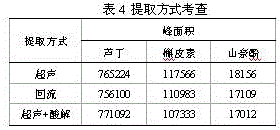
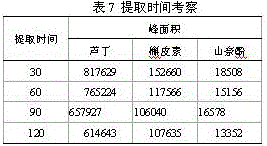
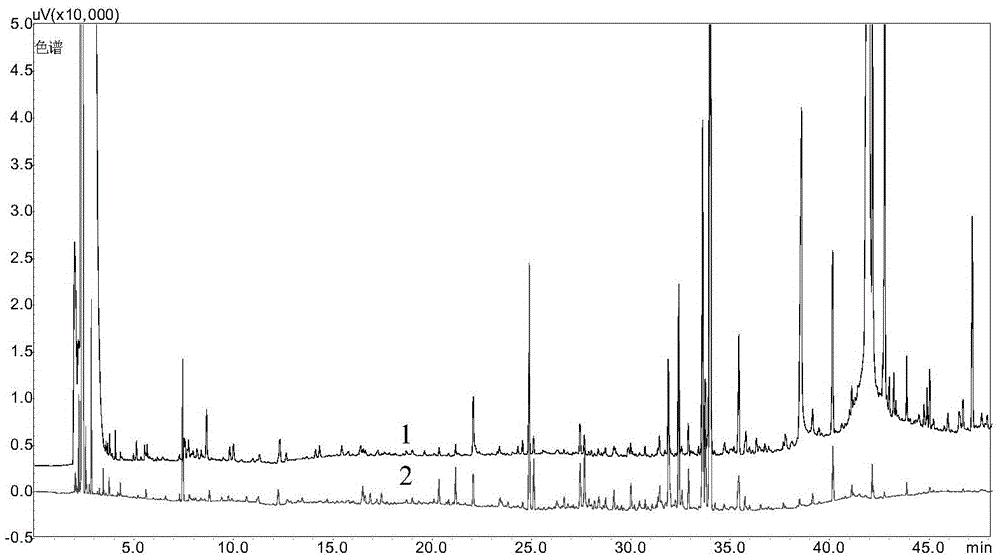
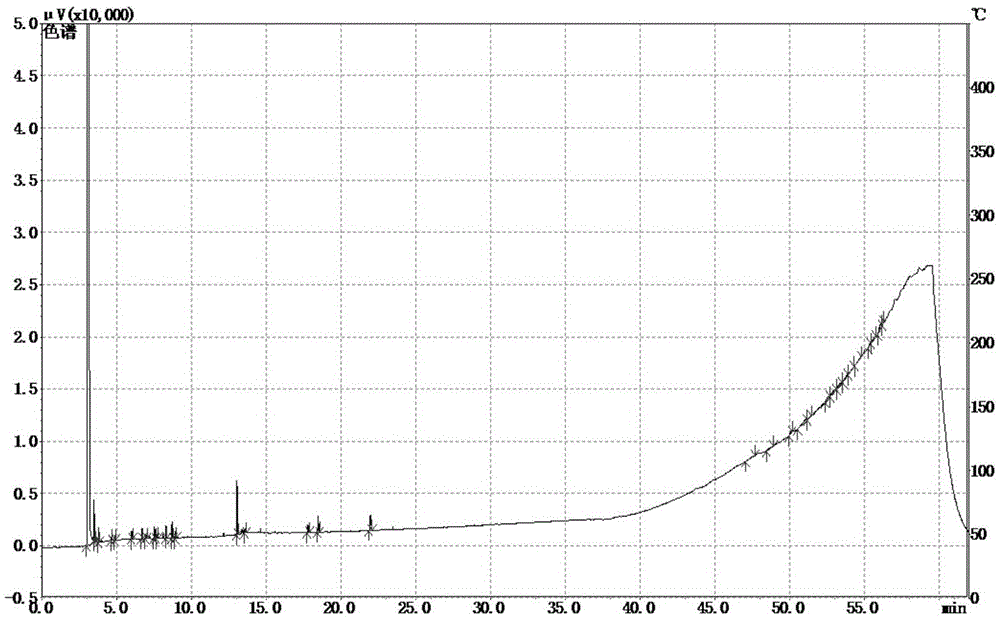
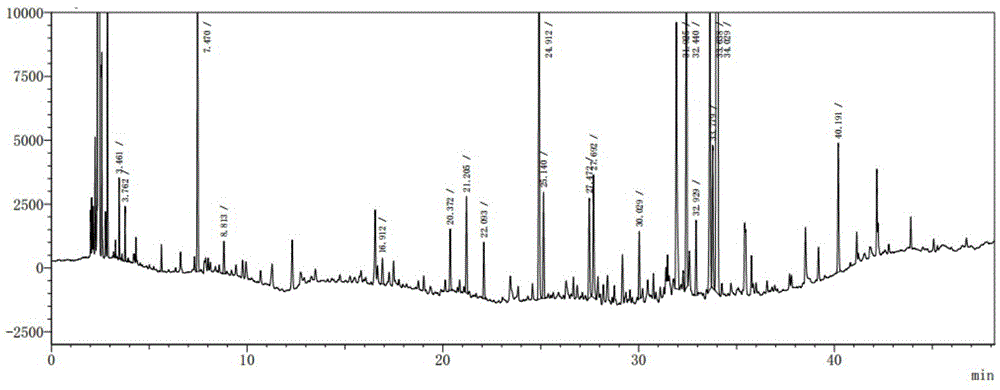
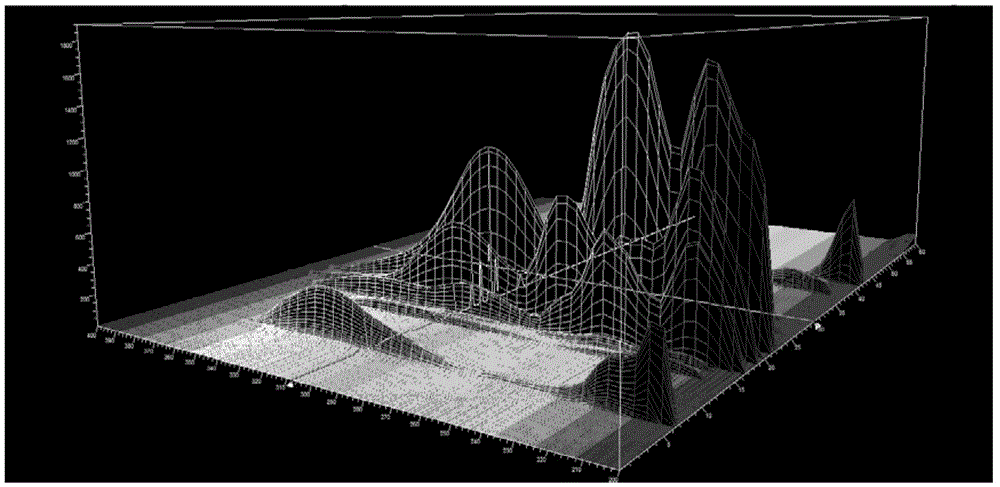
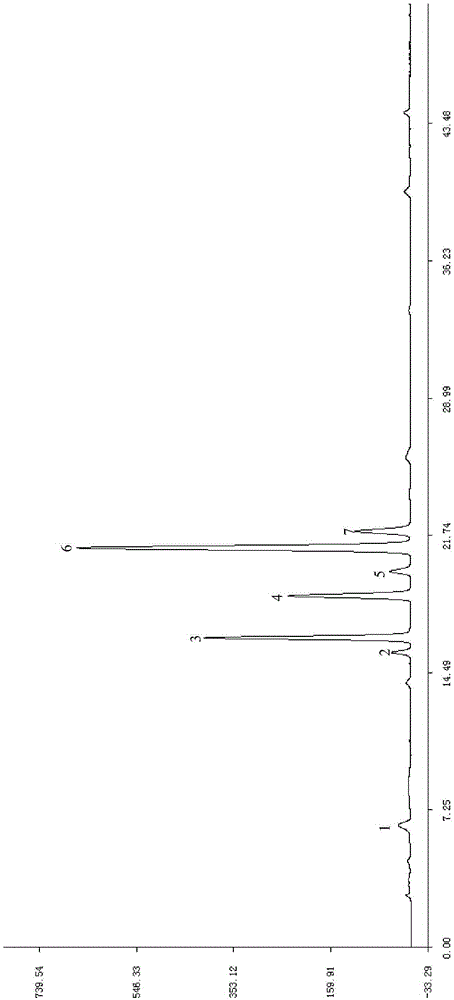
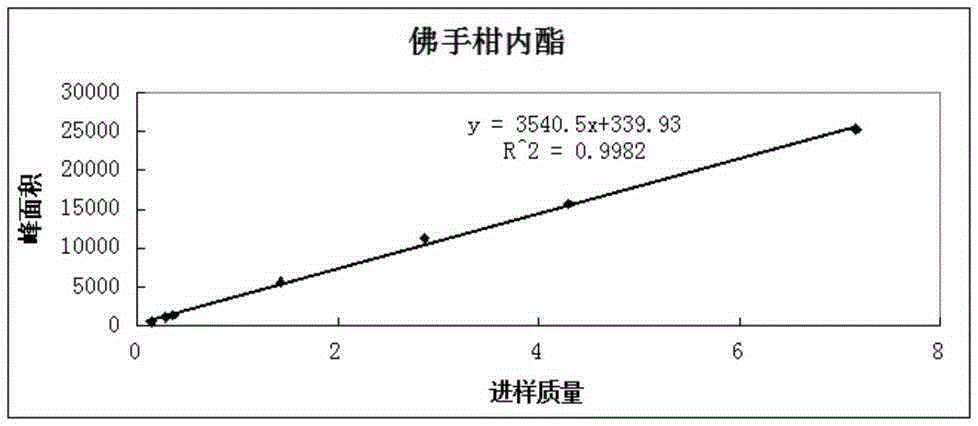
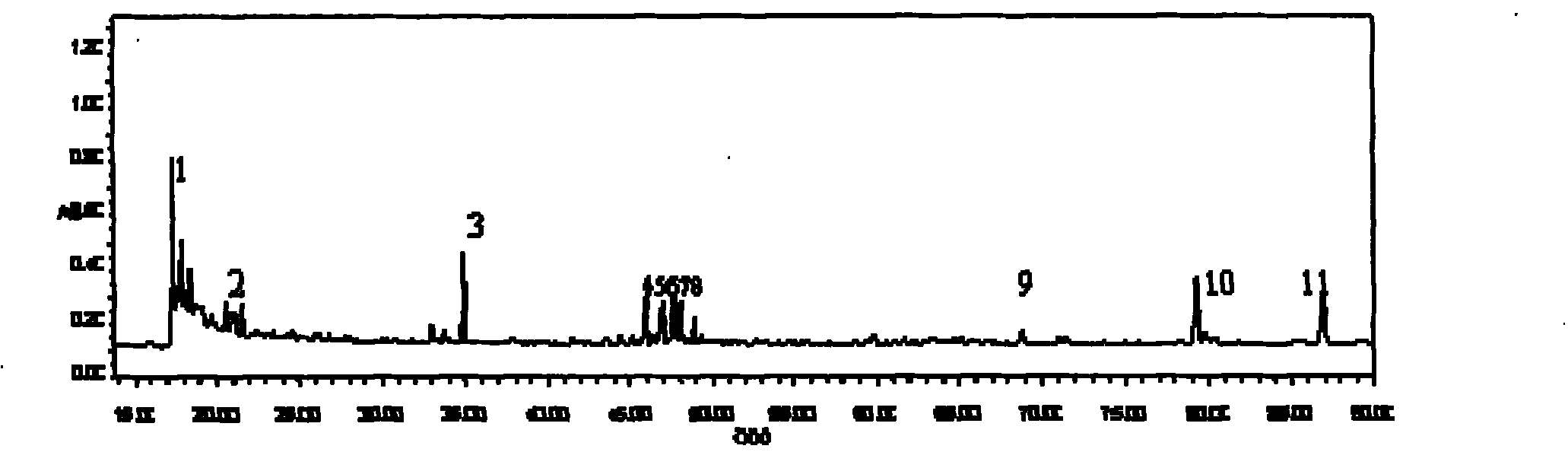
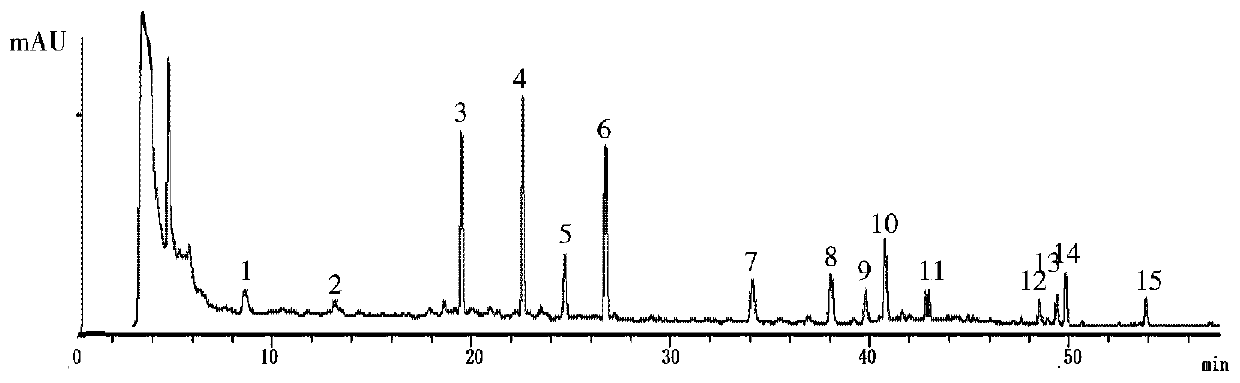
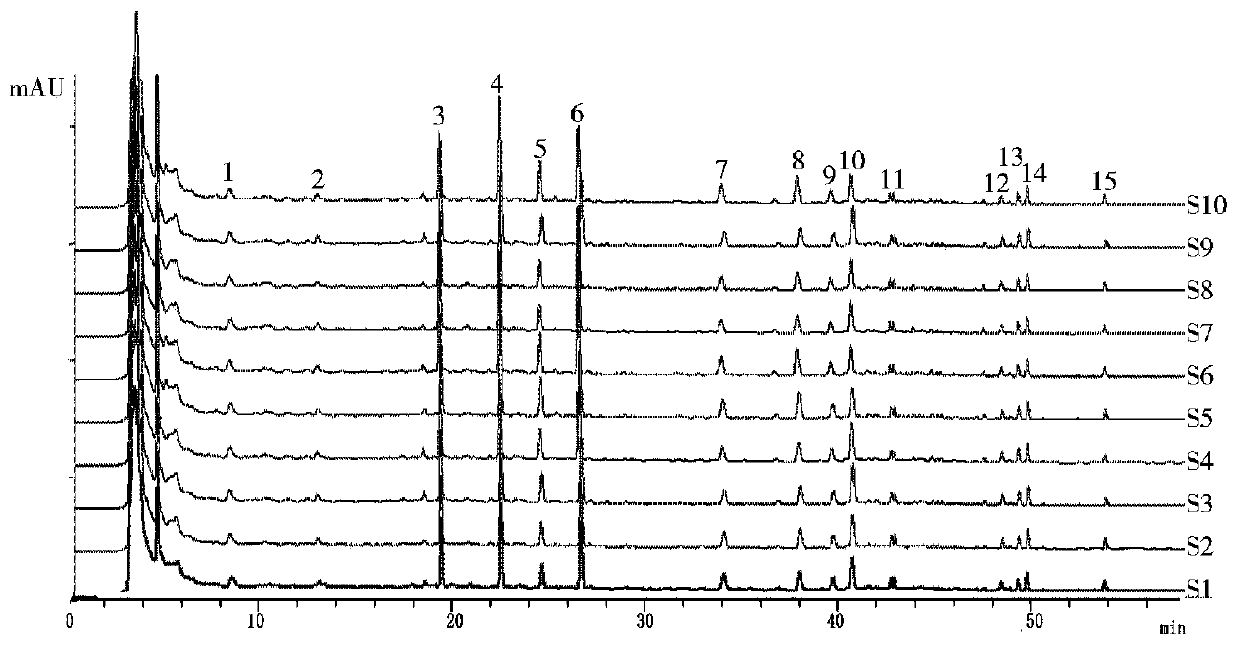
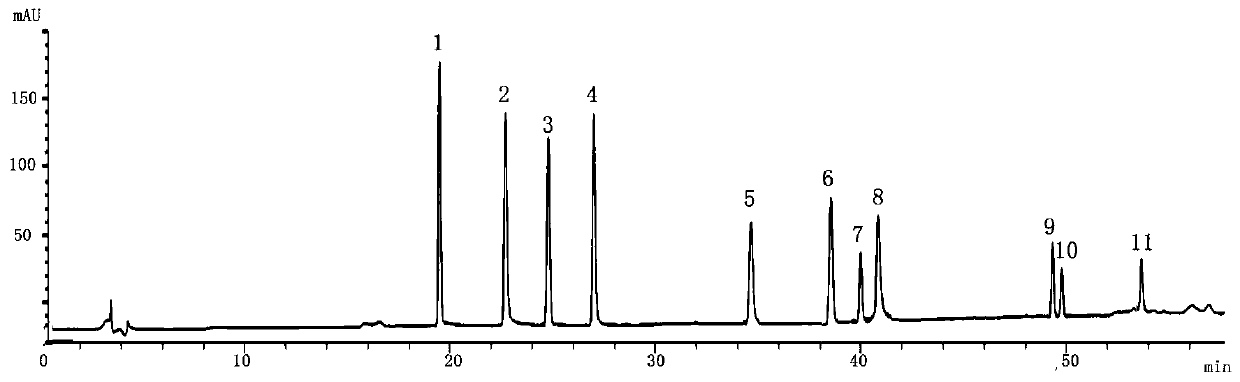
Patents
Literature
53results about How to "Many peaks" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
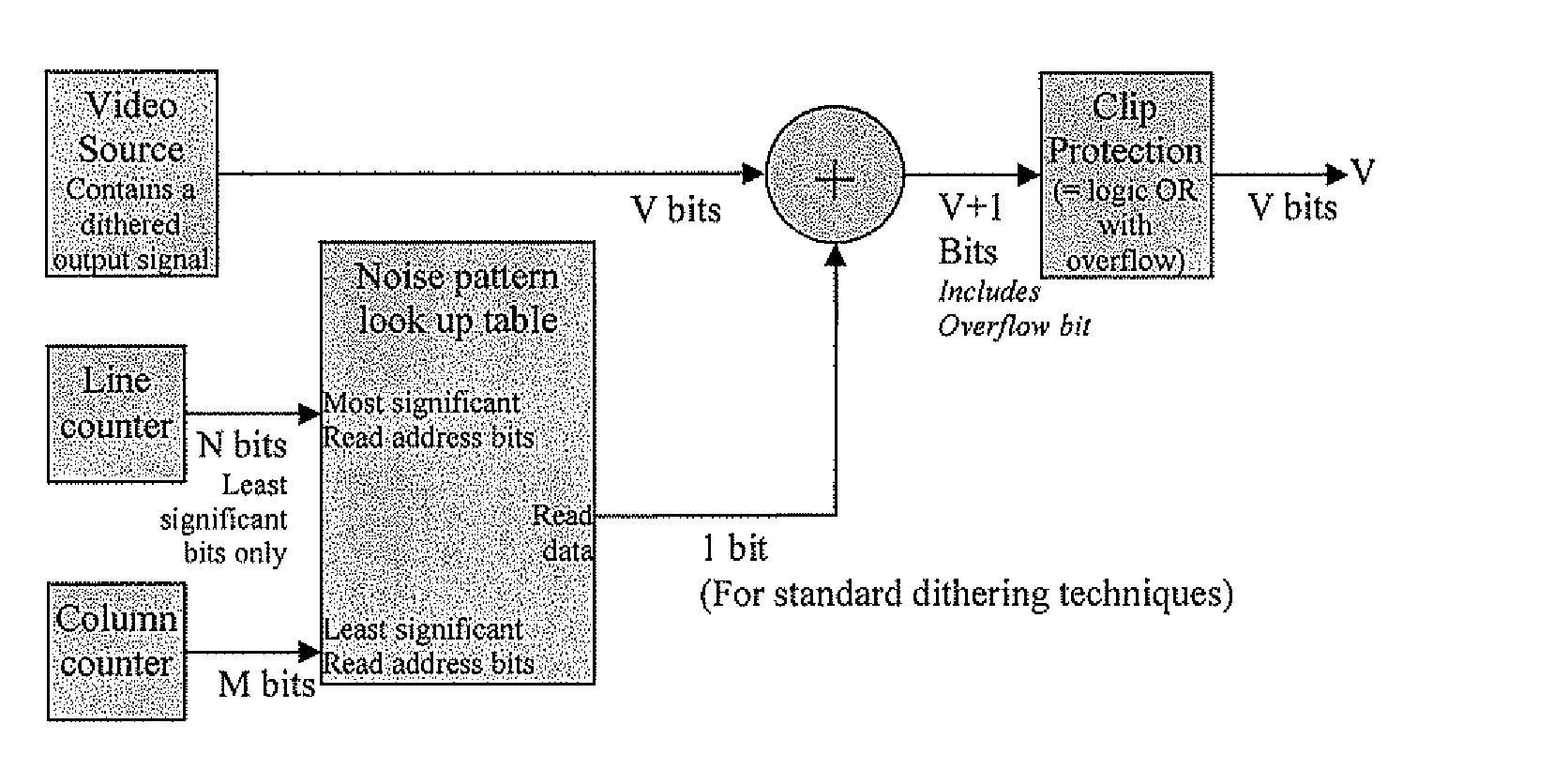
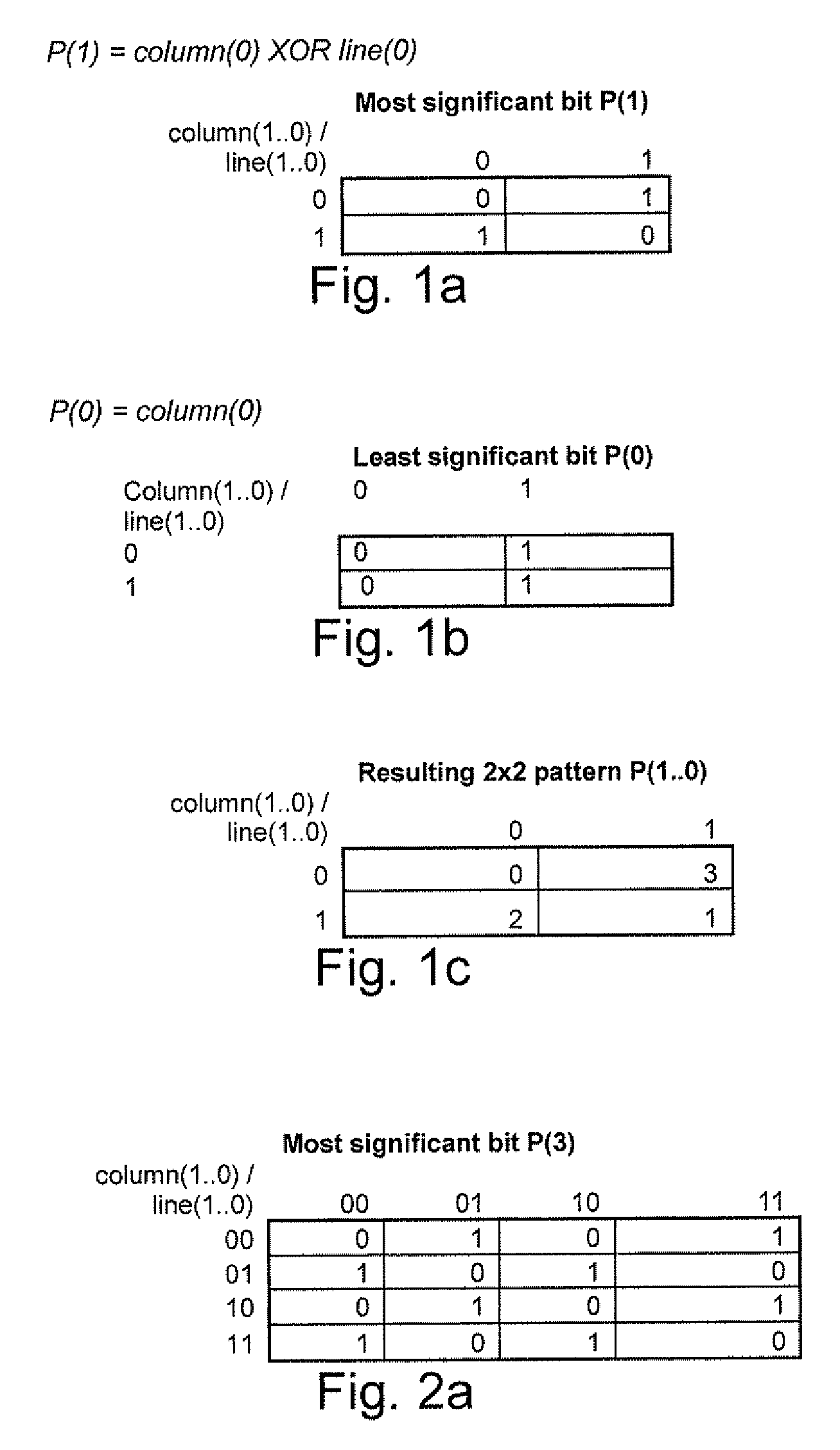
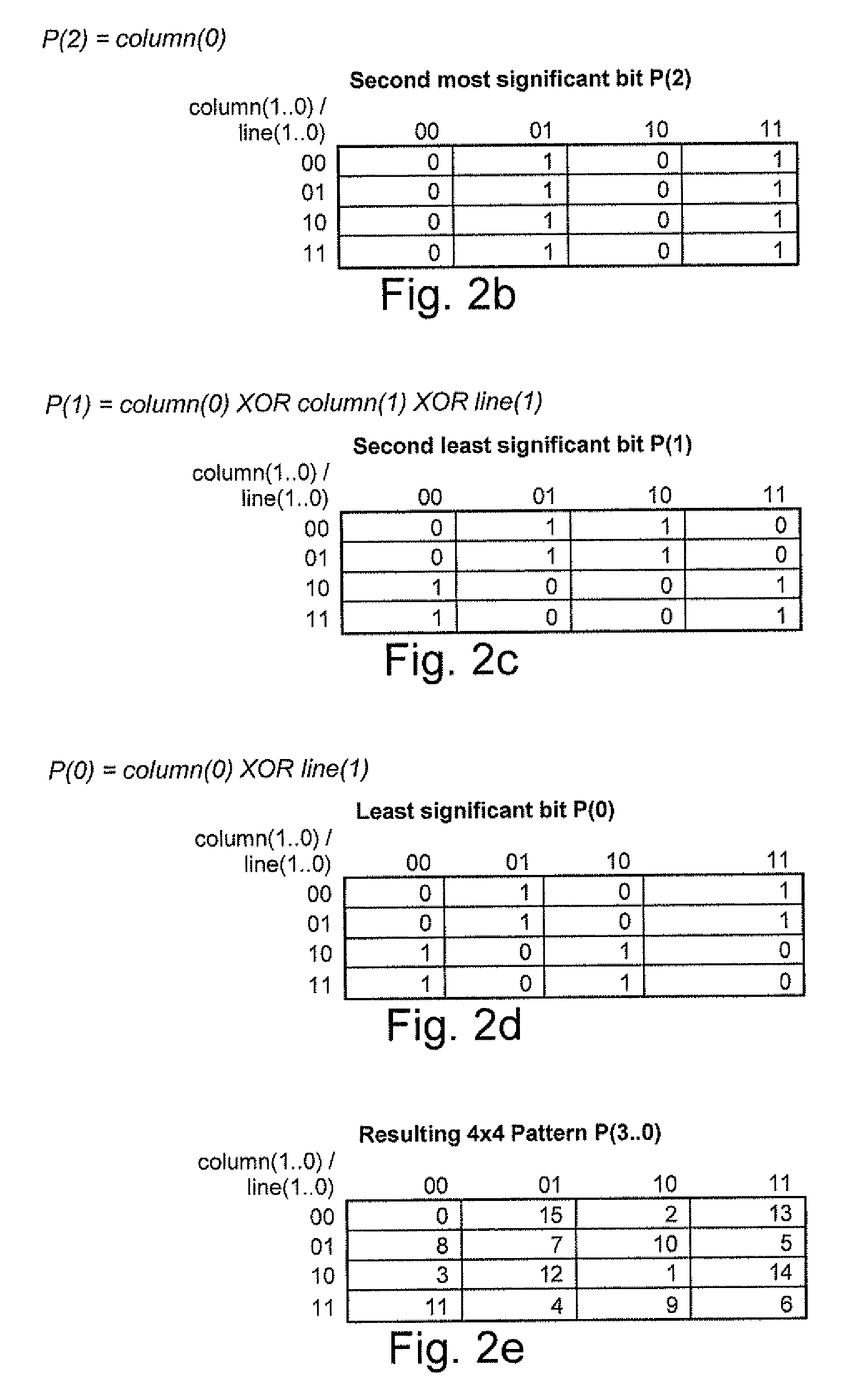
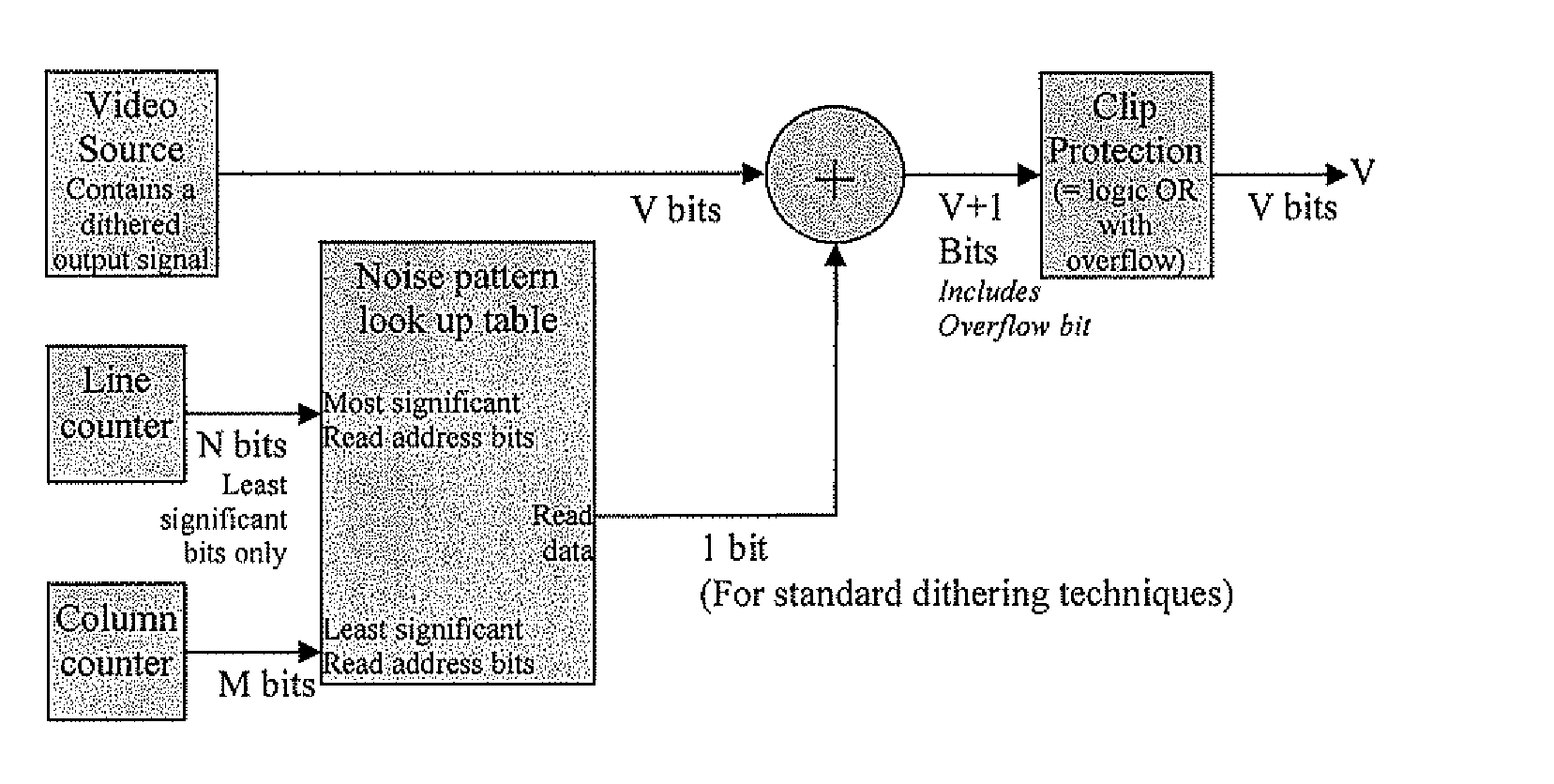
Method and device to enhance image quality in digital video processing systems using dithering
ActiveUS20100259553A9Speed up the processSuitable for processingTelevision system detailsTexturing/coloringPattern recognitionDigital video
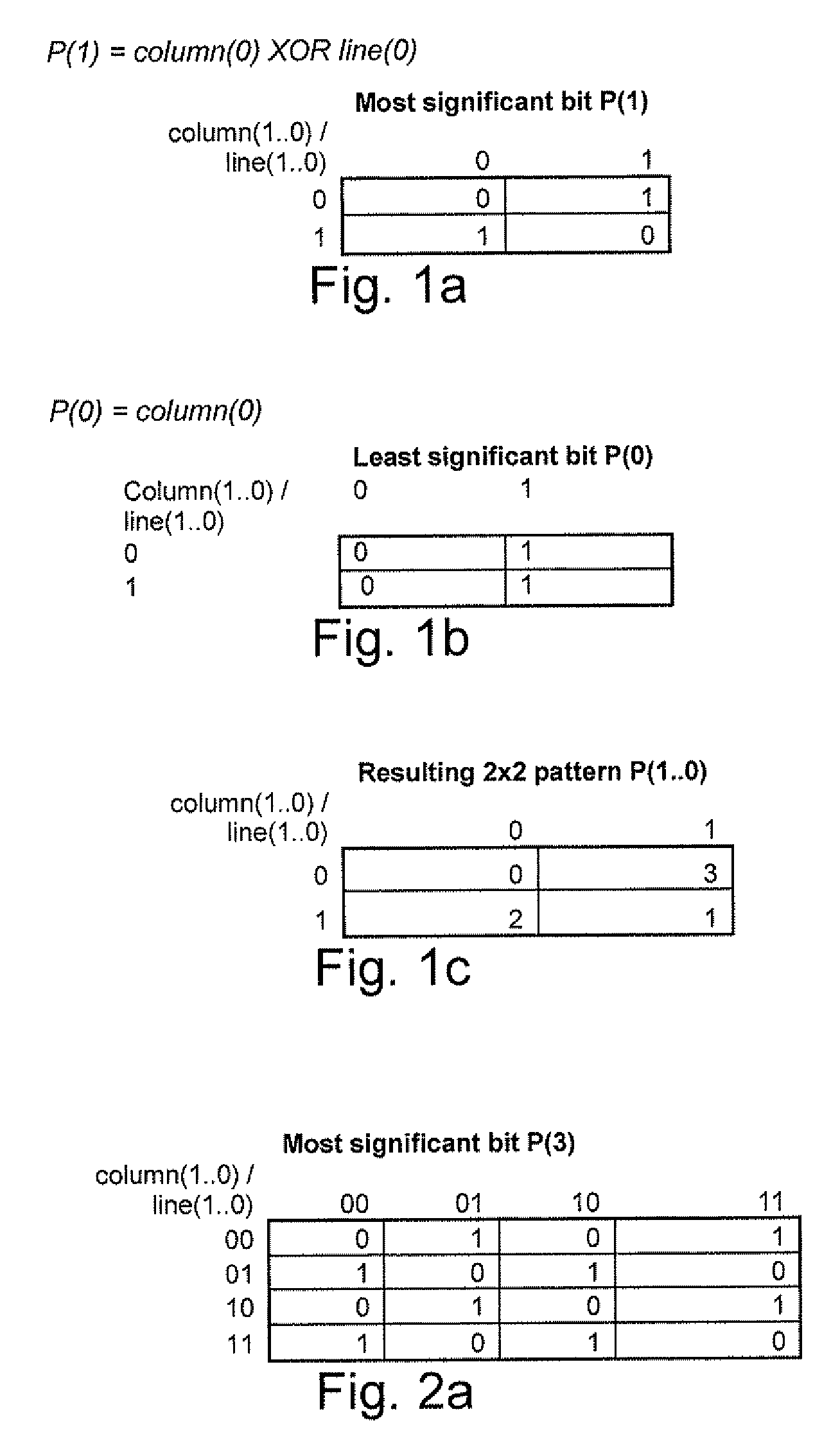
A processing chain for a digital image signal (12) applies a dither pattern (14), having a first spectrum, to the image signal at a point in the processing chain. A further noise pattern (10) is applied to the image signal during the processing chain. The noise pattern (10) has a second spectrum which is configured such that the combination of the first spectrum and second spectrum results in a more continuous spectrum. Another aspect describes a noise pattern (10) which can be used as an offset dither pattern for digital images, especially before colour bit depth reduction. The noise pattern comprises an array of values which are linearly distributed across a range, with each value in the range occurring an equal number of times. Similar values at extreme ends of the range of values are dispersed within the array. The pattern has a Poisson-disk two-dimensional spectral energy distribution. Values are positioned in the array based on distance to similar values in neighbouring repetitions of the array. The array has “magic square” properties.
Owner:BARCO NV
Canzhiling oral solution fingerprint map building method, fingerprint map and application thereof
ActiveCN104849364AImprove stabilityGood reproducibilityComponent separationSystems analysisChemical composition
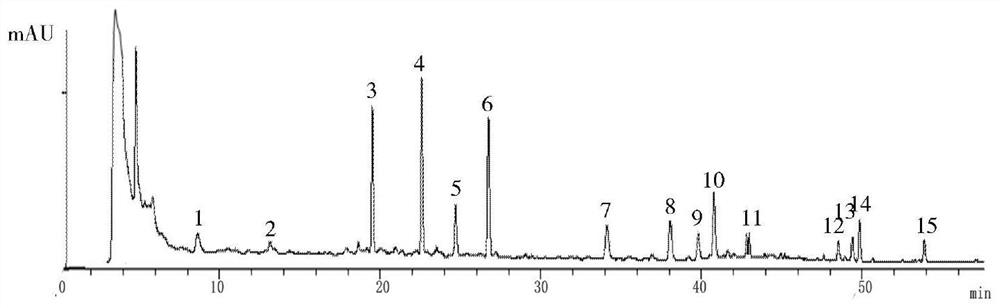
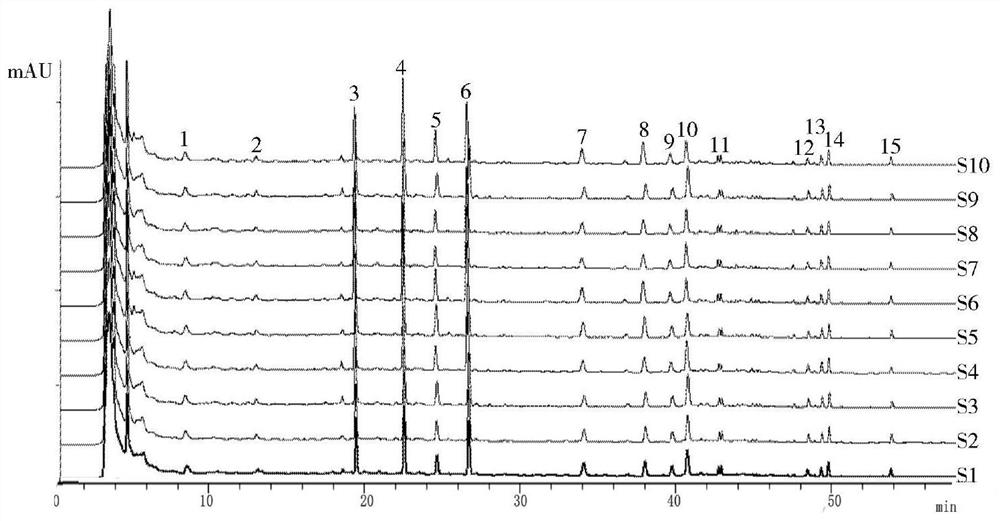
The invention discloses a canzhiling oral solution fingerprint map building method. The map includes the following steps of preparation of a test solution, preparation of a reference solution, testing through a high performance liquid chromatograph and processing of data and a map. The invention further discloses a Canzhiling oral solution fingerprint map and a method for utilizing the fingerprint map to control quality of a Canzhiling oral solution. The Canzhiling oral solution fingerprint map building method is simple in operation, stable, reliable, high in accuracy and high in separation degree, the fingerprint map is high in stability and reproducibility and large in information quantity, and the fingerprint map is adopted as a quality control means for the Canzhiling oral solution, so that one-sidedness caused by judging of overall quality of a preparation by testing one or two chemical ingredients is avoided, and probability of artificial processing in order to enabling quality to be up to standards is lowered; samples of multiple batches are analyzed systematically, so that quality of the Canzhiling oral solution can be evaluated more comprehensively and scientifically, and product quality and efficacy are guaranteed.
Owner:SHANDONG UNIV
Fingerprint spectrum detection method for meridian warming decoction
ActiveCN110907553AImprove stabilityGood reproducibilityComponent separationLigusticum chuanxiongGinseng
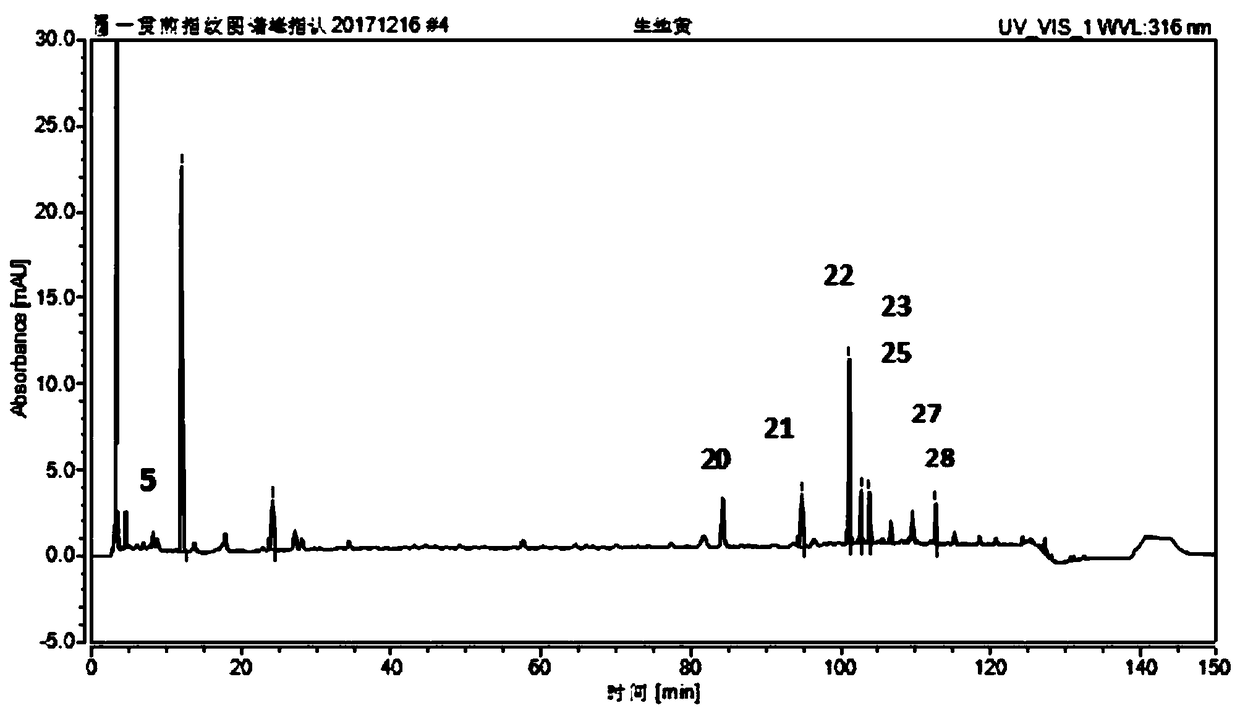
The invention relates to a fingerprint spectrum detection method for meridian warming decoction. The method comprises the following steps: 1) preparing a test solution; weighing 1-2g of angelica sinensis, 1-2g of ligusticum wallichii, 1-2g of radix paeoniae alba, 1-2g of cinnamon, 1-2g of moutan bark, 1-2g of curcuma zedoary powder, weighing 3-4g of ginseng powder, 3-4g of liquorice powder and 3-4g of radix achyranthis bidentatae powder; adding 250-350mL of water, uniformly mixing, heating to boil with strong fire, slowly decocting with slow fire, filtering while the decoction is about 150-170mL, and adding water into the filtrate to dilute to 240-260mL; precisely sucking 8-12mL of the standard decoction of the meridian warming decoction, adding methanol to dilute to 40-60mL, sealing, weighing the mass, carrying out ultrasonic (the power is 180-220W and the frequency is 40-60kHz) treatment for 8-12min, standing overnight, weighing the mass again, supplementing the lost mass with methanol, uniformly shaking, filtering, and taking the subsequent filtrate, thereby obtaining the test solution; 2) detecting: taking and injecting 5-15 [mu]L of the test solution obtained in the previous step into a high performance liquid chromatograph, and obtaining a chromatogram; and 3) performing result judgment: comparing the chromatogram obtained in the previous step with a standard control fingerprint, and if the similarity is greater than 90%, the sample being qualified.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
HPLC fingerprint identification method for origin ginseng protection
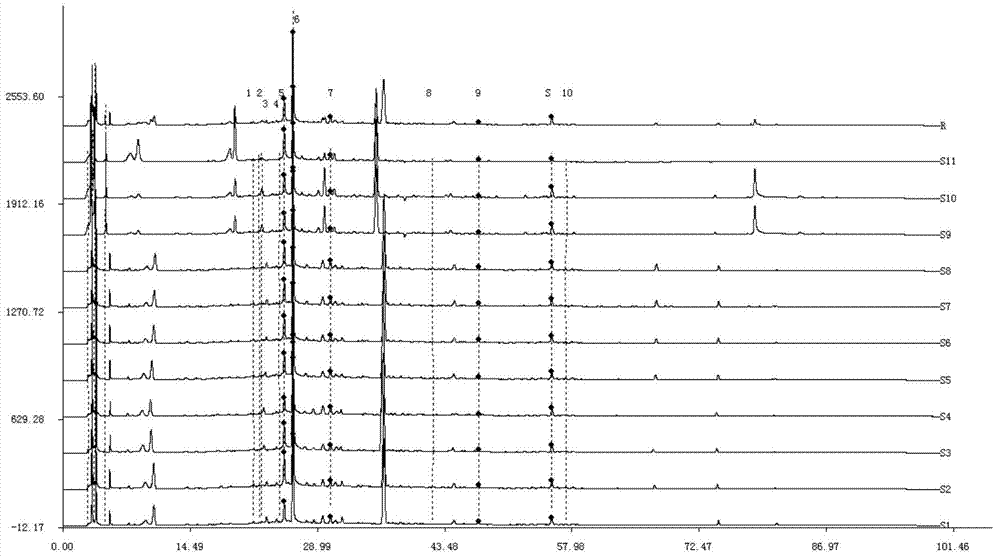
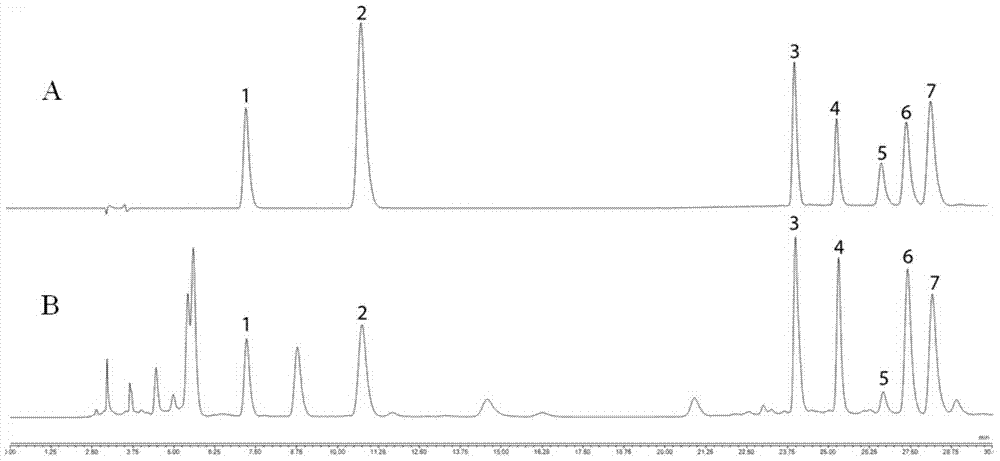
InactiveCN101419200AThe preprocessing method is simpleMany peaksComponent separationHplc fingerprintMedicinal herbs
The invention provides a method for identifying HPLC fingerprints for protecting ginseng of origin, which is characterized by comprising the following steps: A, establishing liquid phase chromatograph fingerprints of the ginseng of origin, namely using the ginseng in the same habitat as a standard, analyzing the ginseng by liquid phase chromatograph and establishing fingerprints; and B, detecting a sample, namely taking a ginseng sample to be detected, detecting the sample under the same conditions as the standard respectively to obtain a spectrogram of the detected sample, and analyzing the fingerprints of the detected sample and the standard by a direct observational method or fingerprints software as qualitative basis. The method has simple pretreatment for fingerprints of the ginseng medicinal material established by adopting the liquid phase chromatograph, multiple peak numbers and better separation degree, therefore, contents of main chemical compositions of the ginseng medicinal material have large difference caused by different growing geographic environments and climates. The HPLC fingerprints of the ginseng can provide reference for distinguishing high-end ginseng health-care products from origin places and non-origin places, and provide reference for establishing fingerprints of other traditional Chinese medicinal materials. The research of methodology shows that all the precision, the stability and the repeatability have better application prospect.
Owner:HEBEI UNIVERSITY
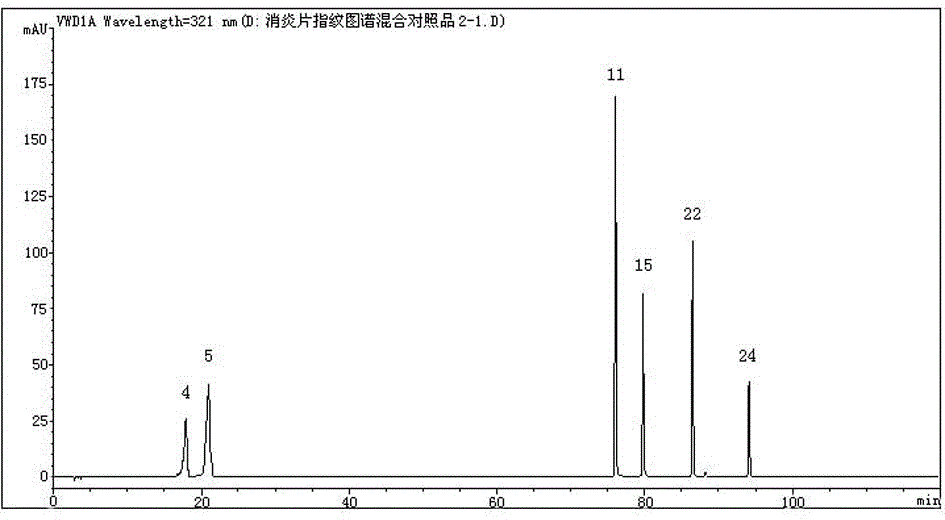
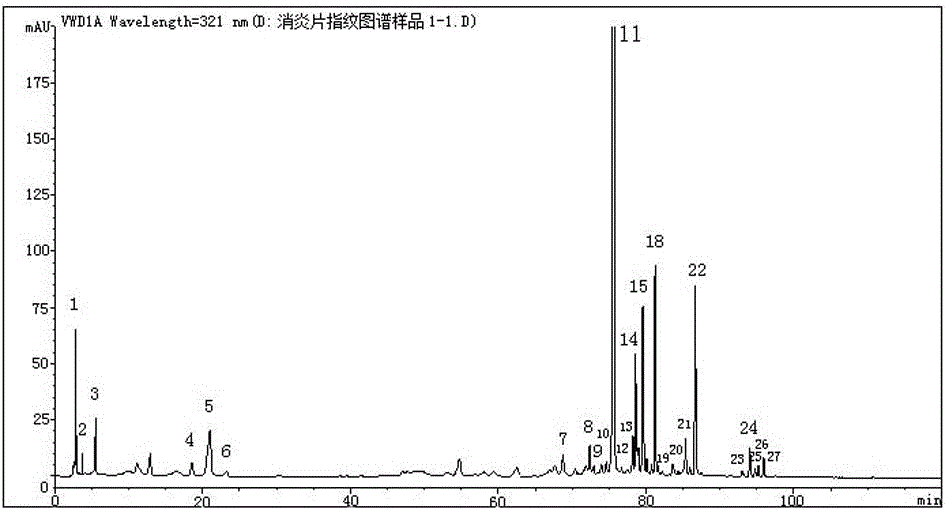
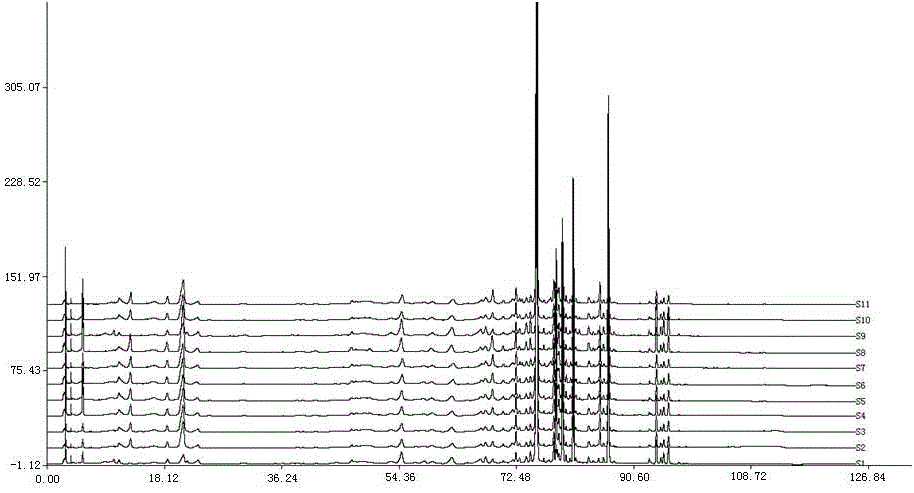
Anti-inflammatory tablet HPLC fingerprint construction method
An anti-inflammatory tablet HPLC fingerprint construction method comprises the following steps: 1, preparing a test solution: grinding an anti-inflammatory tablet, weighing the ground tablet, placing the ground tablet in an extractor, adding petroleum ether, carrying out hot reflux extraction, removing the petroleum ether, adding methanol, carrying out ultrasonic treatment, supplementing methanol, and filtering the obtained solution; 2, preparing a reference solution: taking a chlorogenic acid reference substance, an aesculetin reference substance, a scutelloside reference substance, a linarin reference substance, a baicalein reference substance and a wogonin reference substance; and 3, determining: respectively taking the reference solution and the test solution, respectively injecting the reference solution and the test solution to a liquid chromatograph, recording the chromatogram in 120min, and processing the chromatogram through using fingerprint software to obtain the fingerprint of the anti-inflammatory tablet. The method has the advantages of establishing the common mode of the HPLC characteristic fingerprint of the anti-inflammatory tablet, calibration of 27 common peaks, effective characterization of the quality of the anti-inflammatory tablet, overcoming of unicity and one-sidedness of original quality control methods, and high application values.
Owner:吉林修正药业新药开发有限公司 +1
Preparation method of natural high-concentration black tea essence
ActiveCN104336575AKeep it authenticOvercoming the disadvantages of low concentrationTea flavoringFood preparationHigh concentrationBlack tea
The invention discloses a preparation method of a natural high-concentration black tea essence. The method comprises the following steps: (1) tea leaves are added into a sealed extraction tank with a steam heating and condensing device; steam is delivered in for distillation; a condensate is collected, such that black tea distillate liquid A is obtained; (2) the liquid A is collected, and when the weight of the liquid A reaches 2-6 times that the weight of the raw material (tea leaves), collection is stopped, and the liquid A is refrigerated; (3) the obtained liquid A is extracted with an SCC (spinning cone column) method; and through adjusting extraction parameters, the natural high-concentration black tea essence B is obtained. According to the invention, distillation extraction is carried out with the combination of a steam distillation method and the SCC (spinning cone column) method, such that a series of high-concentration water-soluble black tea essence can be obtained. The product is clear and transparent, and has the advantages of pure and natural aroma and true taste. The product is suitable for beverages, dairy products, and ice products. The product is an upgrade and replacement of traditional food flavors. The materials are natural, and the process is advanced, such that current consumption demands of naturalness and healthiness can be satisfied.
Owner:SHANGHAI AIPU VEGETABLE TECH +1
Method for detecting quality of sanajon oral solution with qualitative and quantitative evaluation
The invention discloses a HPLC fingerprint detection method of a sanajon oral solution. The fingerprint has the advantages of more appearance, good resolution, good repeatability and high precision, and provides effective guarantee to comprehensive monitor on the quality of a sanajon oral solution. The extraction method and chromatographic condition of the fingerprint detection method are utilizedto detect content of five active ingredients in the sanajon oral solution, including gallic acid, allocatechine, picatechin, corilagin and ellagic acid, and realizes qualitative and quantitative researches on the sanajon oral solution.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method for simultaneous detection of iridoid glycoside, phenylethanoid glycoside, flavone and dicaffeoyl ingredients in lamiophlomis rotata
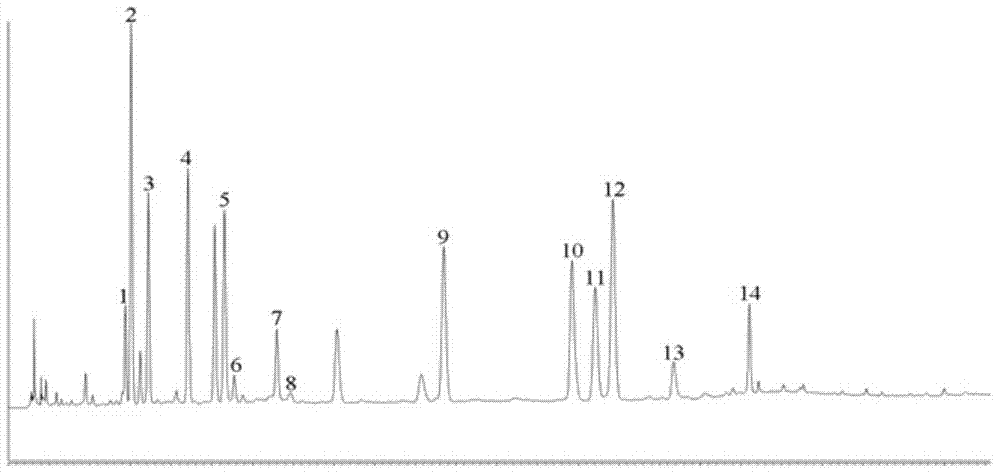
InactiveCN104237441AComprehensive and more accurate quality controlMany peaksComponent separationLamiophlomis rotataMaterial quality
The invention provides a method for simultaneous detection of iridoid glycoside, phenylethanoid glycoside, flavone and dicaffeoyl ingredients in lamiophlomis rotate. In the detection method, the peak amount is large, separation degree is high, a base line is stable, the lamiophlomis rotate can be effectively detected, and new selection is provided for guaranteeing of medicine material quality. The iridoid glycoside, phenylethanoid glycoside, flavone and dicaffeoyl ingredients are detected under the same chromatographic condition for the first time, seven specific compounds are identified, and the quality of lamiophlomis rotate medicine materials can be comprehensively and accurately controlled.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method for detecting three kinds of flavonoid constituents in seedless roxburgh rose fruits simultaneously
The invention discloses a method for detecting three kinds of flavonoid constituents in seedless roxburgh rose fruits simultaneously. The method comprises the steps that seedless roxburgh rose fruit dry powder to be detected is obtained, methyl alcohol or water-containing methyl alcohol is used for extraction, filtrate decompression and rotary drying are carried out, ultrapure water is added, normal butanol is saturated with water, extraction is carried out two times according to the ethanol and water ratio of 4:1, then, extraction is carried out two times through ethyl acetate, filtrates are combined and subjected to decompression and rotary drying, the volume is made constant, and a test solution is prepared; rutin, quercetin and kaempferol are selected as comparison products for preparing a comparison solution; the comparison solution and the test solution are sample and analyzed in a high performance liquid chromatograph under the same chromatographic condition, and the three kinds of flavonoid constituents in seedless roxburgh rose fruits are monitored according an external standard method. The main flavonoid constituents in seedless roxburgh rose fruits are detected for the first time under the same chromatographic condition, three specific compounds are identified, and the method can be more comprehensively and accurately used for intensive study of flavonoid active constituents in seedless roxburgh rose fruits.
Owner:GUIZHOU NORMAL UNIVERSITY
Construction method of specific chromatogram of volatile components in Zhengtian pill preparation and detection method of volatile components in Zhengtian pill preparation
ActiveCN105548411AImprove quality controlImprove integrityComponent separationWater vaporRetention time
The invention relates to a construction method of a specific chromatogram of volatile components in a Zhengtian pill preparation and a detection method of the volatile components in the Zhengtian pill preparation. According to the construction method and the detection method, a water steam distillation method is used for extracting the volatile components in the Zhengtian pill preparation, the gas chromatography is used for performing specific chromatogram analysis on the volatile components, the relative retention time ratio is used as an evaluation index, the specific chromatogram of the volatile components in the Zhengtian pill preparation is built, and detection is performed on the Zhengtian pill preparation. The construction method and the detection method are good in repeatability, the characteristic peak separation degree is high, and method references are provided for overall quality control over the Zhengtian pill preparation.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
HPLC finger-print detection method of Heracleum souliei
The invention discloses a HPLC finger-print detection method of Heracleum souliei. The finger-print has multiple peaks, good resolution, good repeatability and high precision, and thereby provides an effective guarantee for comprehensive monitor of Heracleum souliei quality. By means of an extraction method and a chromatogram condition of the finger-print detection method, contents of two active components psoralen and bergapten in Heracleum souliei can also be detected, so that quality of Heracleum souliei can be controlled from more aspects according to the invention.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Raw fleece-flower root HPLC fingerprint detection method
The invention discloses a raw fleece-flower root HPLC fingerprint detection method. The detection method comprises the following steps: (1) preparing a control substance solution; (2) preparing a reference substance solution; (3) establishing a control fingerprint; (4) preparing a test article solution; (5) detecting the test article solution. The obtained fingerprint has the advantages that there are many peaks, the resolution and repeatability are good, the precision is high, and the quality of raw fleece-flower root can be effectively monitored. By using the extraction method and chromatogram conditions of provided fingerprint detection method, contents of 16 components of raw fleece-flower root can be measured, and thus the quality of raw fleece-flower root can be monitored from more aspects.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method and device to enhance image quality in digital video processing systems using dithering
ActiveUS20090225097A1Speed up the processSuitable for processingTelevision system detailsTexturing/coloringPattern recognitionDigital video
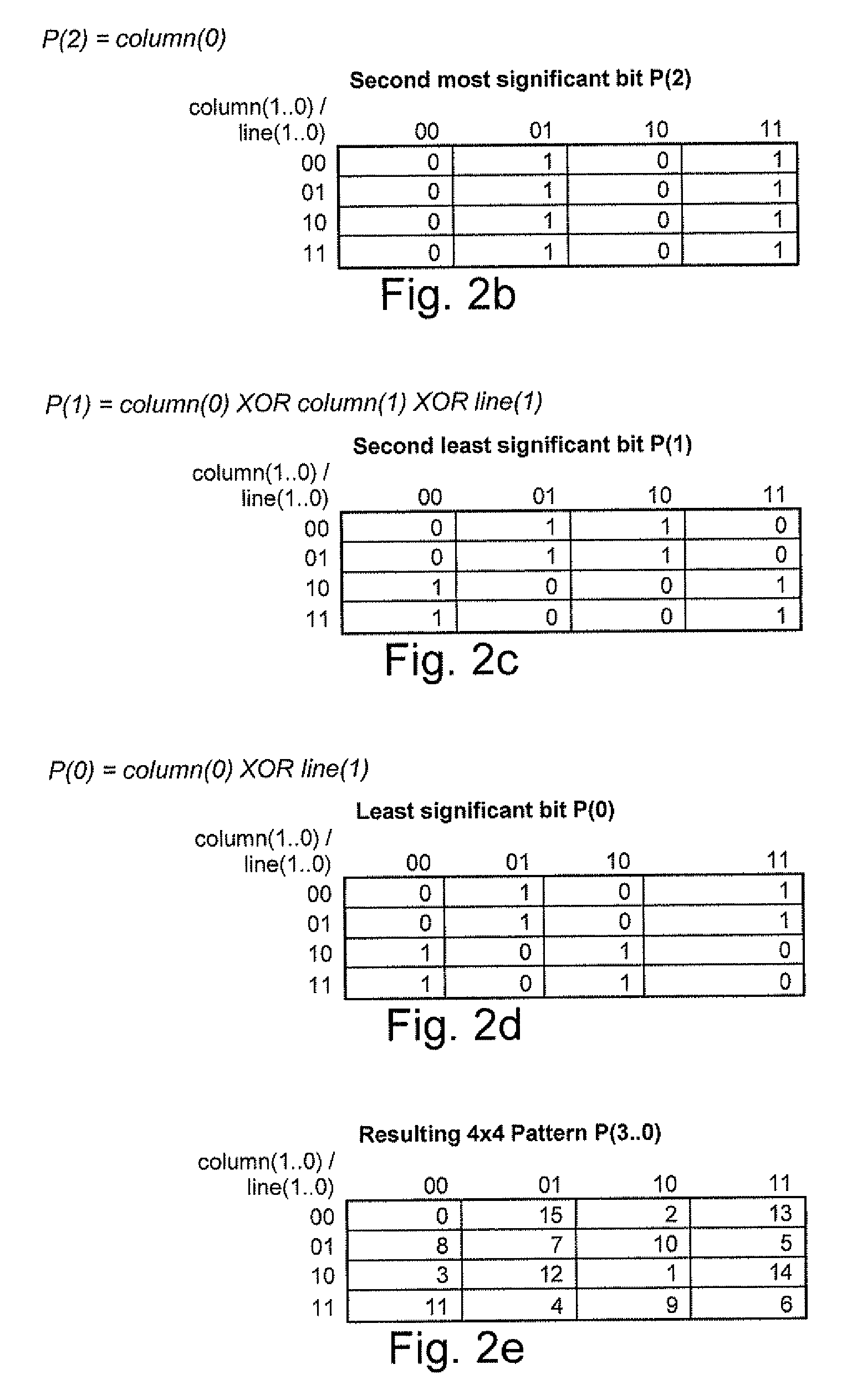
A processing chain for a digital image signal (12) applies a dither pattern (14), having a first spectrum, to the image signal at a point in the processing chain. A further noise pattern (10) is applied to the image signal during the processing chain. The noise pattern (10) has a second spectrum which is configured such that the combination of the first spectrum and second spectrum results in a more continuous spectrum. Another aspect describes a noise pattern (10) which can be used as an offset dither pattern for digital images, especially before colour bit depth reduction. The noise pattern comprises an array of values which are linearly distributed across a range, with each value in the range occurring an equal number of times. Similar values at extreme ends of the range of values are dispersed within the array. The pattern has a Poisson-disk two-dimensional spectral energy distribution. Values are positioned in the array based on distance to similar values in neighbouring repetitions of the array. The array has “magic square” properties.
Owner:BARCO NV
HPLC fingerprint identification method for origin ginseng protection
InactiveCN101419200BThe preprocessing method is simpleMany peaksComponent separationHplc fingerprintPretreatment method
The invention provides a method for identifying HPLC fingerprints for protecting ginseng of origin, which is characterized by comprising the following steps: A, establishing liquid phase chromatograph fingerprints of the ginseng of origin, namely using the ginseng in the same habitat as a standard, analyzing the ginseng by liquid phase chromatograph and establishing fingerprints; and B, detectinga sample, namely taking a ginseng sample to be detected, detecting the sample under the same conditions as the standard respectively to obtain a spectrogram of the detected sample, and analyzing the fingerprints of the detected sample and the standard by a direct observational method or fingerprints software as qualitative basis. The method has simple pretreatment for fingerprints of the ginseng medicinal material established by adopting the liquid phase chromatograph, multiple peak numbers and better separation degree, therefore, contents of main chemical compositions of the ginseng medicinal material have large difference caused by different growing geographic environments and climates. The HPLC fingerprints of the ginseng can provide reference for distinguishing high-end ginseng health-care products from origin places and non-origin places, and provide reference for establishing fingerprints of other traditional Chinese medicinal materials. The research of methodology shows that all the precision, the stability and the repeatability have better application prospect.
Owner:HEBEI UNIVERSITY
Determination method of capsule fingerprint spectrum for dredging collaterals and reducing phlegm
ActiveCN110927302AImprove stabilityGood reproducibilityComponent separationCholic acidSalvianolic acid B
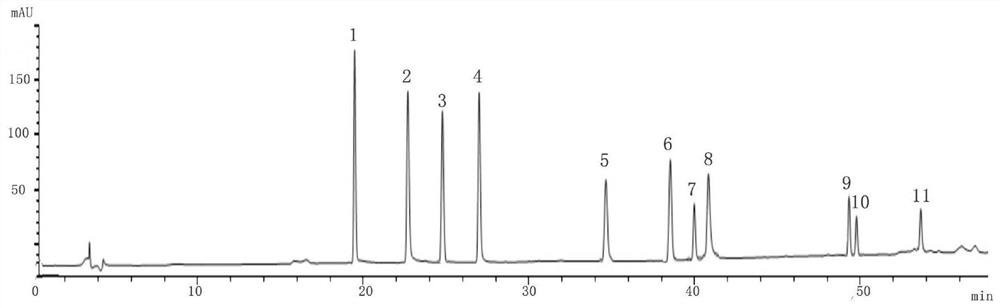
The invention relates to a determination method of a capsule fingerprint spectrum for dredging collaterals and reducing phlegm, which comprises the following steps: (1) test solution prepartion: taking capsule contents for dredging collaterals and reducing phlegm, adding methanol, carrying out ultrasonic extraction, filtering, and taking the subsequent filtrate to obtain the test solution; (2) preparation of reference substance solutions: taking tauroursodeoxycholic acid, gastrodin, sodium tanshinol, tanshinone IIA, salvianolic acid B, protocatechuic aldehyde, ginsenoside Rb1, ginsenoside Rg1,notoginsenoside R1, rheum emodin and rheinic acid reference substances which are dried under reduced pressure to constant weight, and respectively adding methanol to prepare the reference substance solutions; (3) determination: precisely measuring the test solution and the reference solution respectively, injecting the solutions into a high performance liquid chromatograph for determination, andrecording chromatograms; and (4) performing comparison according to the chromatogram and the standard comparison fingerprint, and determining that the product is qualified if the chromatogram and thestandard comparison fingerprint are consistent.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
The establishment method of the fingerprint of Shenzhiling Oral Liquid, its fingerprint and its application
ActiveCN104849364BImprove stabilityGood reproducibilityComponent separationBiochemical engineeringInformation quantity
The invention discloses a canzhiling oral solution fingerprint map building method. The map includes the following steps of preparation of a test solution, preparation of a reference solution, testing through a high performance liquid chromatograph and processing of data and a map. The invention further discloses a Canzhiling oral solution fingerprint map and a method for utilizing the fingerprint map to control quality of a Canzhiling oral solution. The Canzhiling oral solution fingerprint map building method is simple in operation, stable, reliable, high in accuracy and high in separation degree, the fingerprint map is high in stability and reproducibility and large in information quantity, and the fingerprint map is adopted as a quality control means for the Canzhiling oral solution, so that one-sidedness caused by judging of overall quality of a preparation by testing one or two chemical ingredients is avoided, and probability of artificial processing in order to enabling quality to be up to standards is lowered; samples of multiple batches are analyzed systematically, so that quality of the Canzhiling oral solution can be evaluated more comprehensively and scientifically, and product quality and efficacy are guaranteed.
Owner:SHANDONG UNIV
HPLC fingerprint detection method for balsam pear leaves
The invention discloses an HPLC fingerprint detection method for balsam pear leaves. The method comprises the steps of preparing a contrast product solution, preparing a reference product solution, establishing a contrast fingerprint spectrum, and preparing and detecting a test product solution. By virtue of screening of multiple processes, multiple components in the balsam pear leaves are effectively separated and detected, the obtained fingerprint spectrum has multiple peaks, the separation degree of the peaks is good, and the method is good in repeatability and high in precision, so that the quality of a balsam pear leaf medicinal material can be monitored easily, conveniently, quickly, comprehensively, accurately and reliably, and the effective guarantee is provided for comprehensively monitoring the quality of the balsam pear leaf medicinal material.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method for determining traditional Chinese medicine all-along decoction fingerprint spectra by means of HPLC (high performance liquid chromatography)
The invention discloses a method for determining traditional Chinese medicine all-along decoction fingerprint spectra by means of HPLC (high performance liquid chromatography). The method includes steps of carrying out gradient elution on all-along decoction specimen solution by a filler chromatographic column, mobile phase A and mobile phase B; carrying out detection by DAD (diode array detectors). Octadecyl silane chemically bonded silica is used as the filler chromatographic column, a mixture of one or two types of acetonitrile and methyl alcohol is used as the mobile phase A, and one of formic acid aqueous solution, acetic acid aqueous solution and phosphoric acid aqueous solution is used as the mobile phase B. The method for determining the fingerprint spectra by means of HPLC has theadvantages of speediness, simplicity, convenience, comprehensiveness, accuracy and reliability.
Owner:合肥创新医药技术有限公司
A method for determining the fingerprint of Tongluohuatan Capsules
ActiveCN110927302BImprove stabilityGood reproducibilityComponent separationCholic acidTanshinone IIA
The present invention relates to a method for determining the fingerprint of Tongluo Huatan Capsules. The steps of the method are as follows: (1) Preparation of the test solution: take the content of Tongluo Huatan Capsules, add methanol, ultrasonically extract, filter, Take the continued filtrate to obtain the test solution; (2) Preparation of the reference solution: take tauroursodeoxycholic acid, gastrodin, danshensu sodium, tanshinone ⅡA, and salvianolic acid B dried under reduced pressure to constant weight. , protocatechualdehyde, ginsenoside Rb1, ginsenoside Rg1, notoginseng saponin R1, emodin, rhein reference substance, respectively add methanol to make reference substance solution; (3) determination: precision measure the test solution and The reference substance solution is injected into a high-performance liquid chromatograph for measurement, and the chromatogram is recorded; (4) according to the chromatogram and the standard control fingerprint, it is compared, and if it is consistent, it is a qualified product.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Non-reducing peptide mapping method of protein
The invention provides a non-reducing peptide mapping method of a protein. The method comprises the following steps: denaturing the protein by using a denaturing agent, carrying out enzymolysis, terminating the reaction with an acid, and performing detection by reversed phase high-performance liquid chromatography. The non-reducing peptide mapping method makes enzymolysis of the protein sufficient, realizes uniform distribution, large number and good resolution of chromatographic peaks in a map obtained by the reversed phase high-performance liquid chromatography detection, allows the chromatogram to have a stable baseline and truly reflect the situation of each peptide fragment obtained after the enzymolysis of the protein, and is of great significance to controlling the quality of the protein.
Owner:CHENGDU KANGHONG BIOTECH
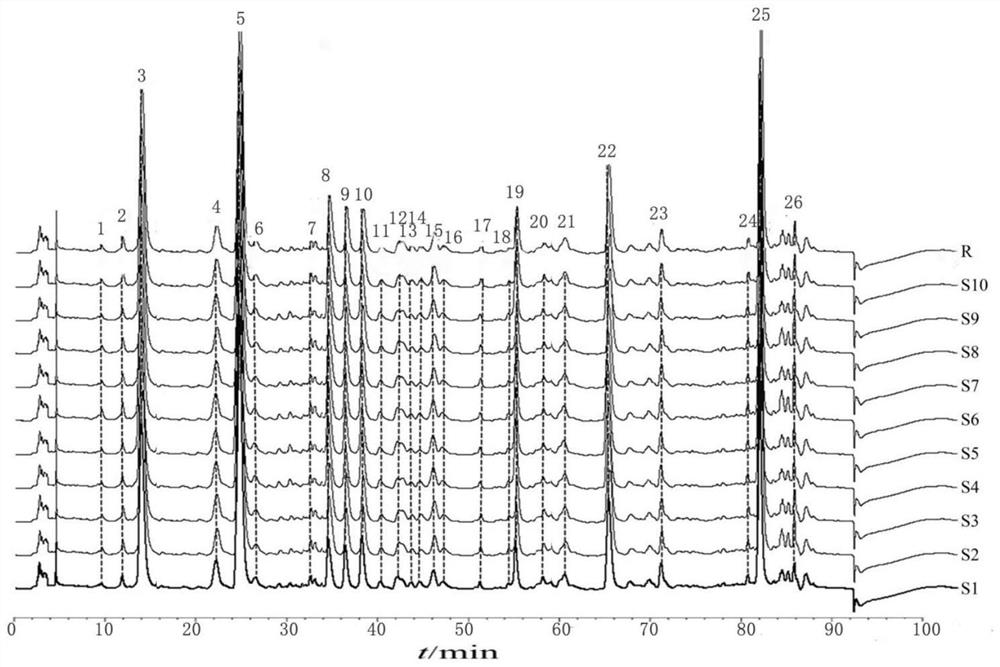
Fingerprint construction method and detection method of angelica sinensis six-yellow decoction composition
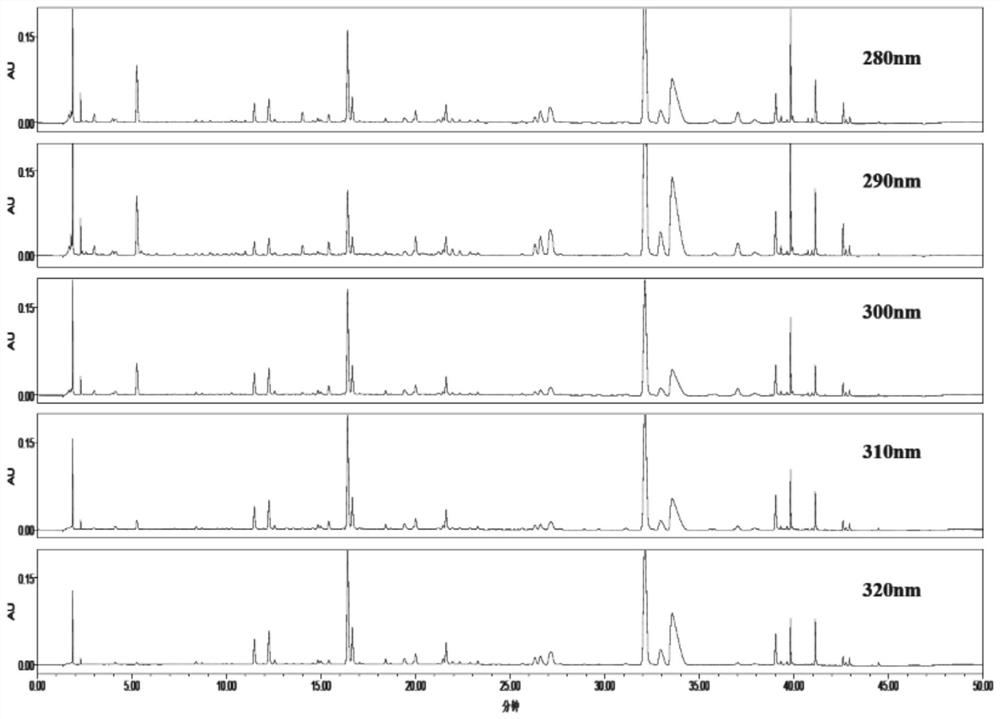
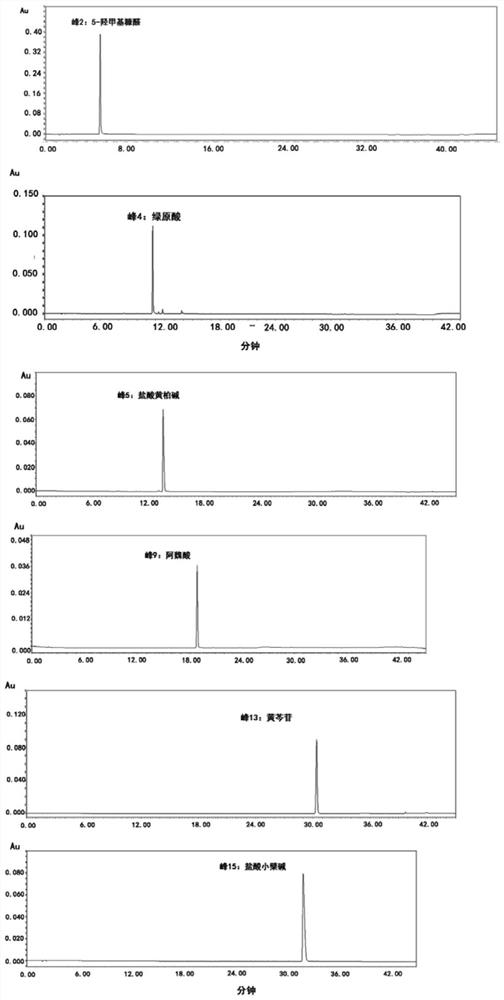
InactiveCN112557553AImprove the level ofEasy to separateComponent separationBiotechnologyChlorogenic acid
The invention discloses a fingerprint construction method and a detection method of an angelica sinensis six-yellow decoction composition, and the construction method comprises the following steps: (1) taking 5-hydroxymethylfurfural, chlorogenic acid, phellodendrine hydrochloride, ferulic acid, baicalin and berberine hydrochloride as reference substances, and respectively preparing reference substance solutions; (2) taking angelica sinensis, radix rehmanniae recen, prepared rehmannia root, scutellaria baicalensis, coptis chinensis, golden cypress and astragalus membranaceus to prepare an angelica sinensis six-yellow decoction composition, and preparing the angelica sinensis six-yellow decoction composition into a test solution; and (3) precisely absorbing the test solution and the reference solutions, injecting the test solution and the reference solutions into a liquid chromatograph for chromatographic analysis with a detection wavelength of 280-320 nm to obtain a test fingerprint anda reference chromatogram, and formulating a standard fingerprint of the angelica sinensis six-yellow decoction composition. The fingerprint construction method can accurately detect the standard fingerprint spectrum of the quality of the angelica sinensis six-yellow decoction composition, and can effectively control the product quality of the angelica sinensis six-yellow decoction preparation.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD
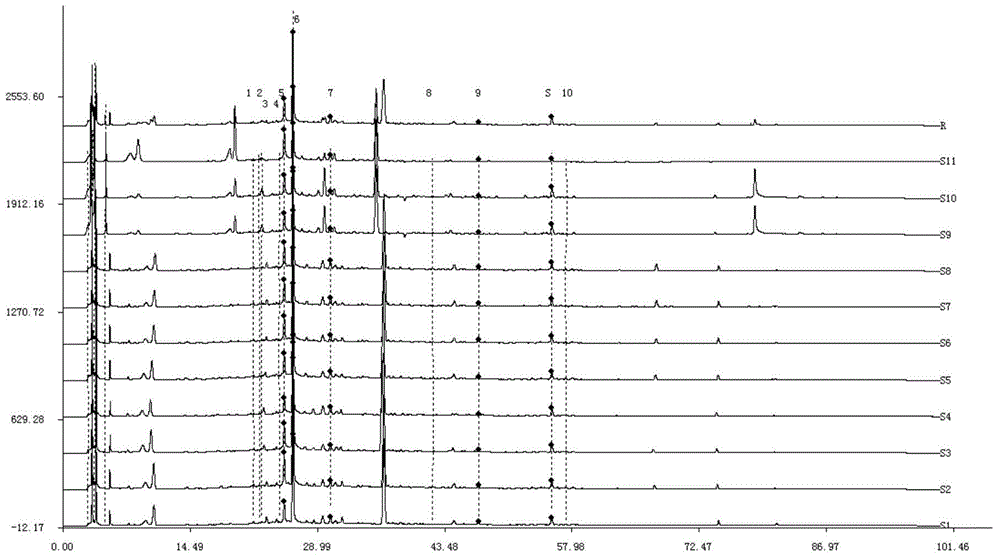
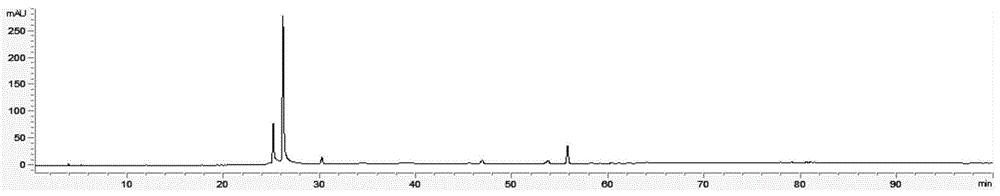
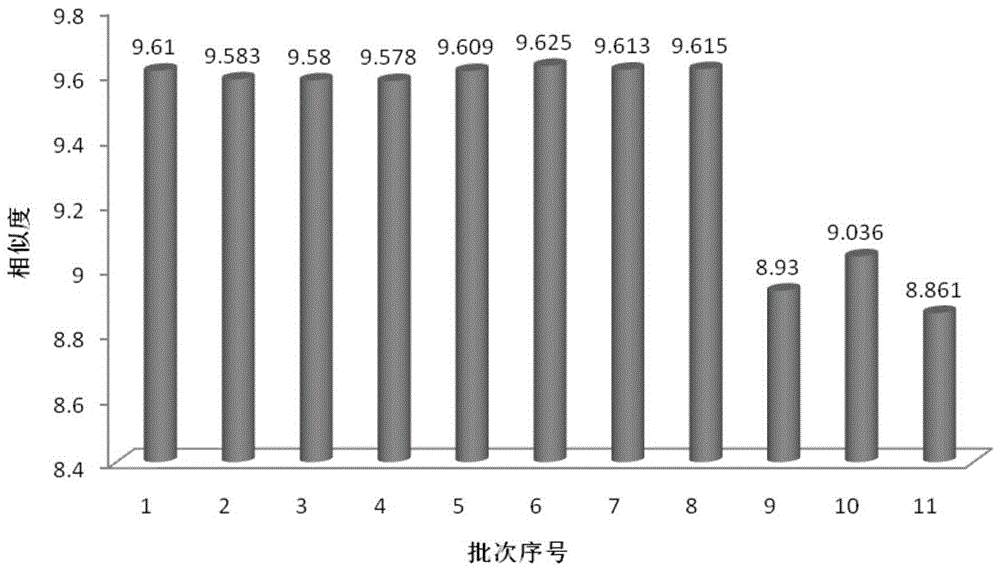
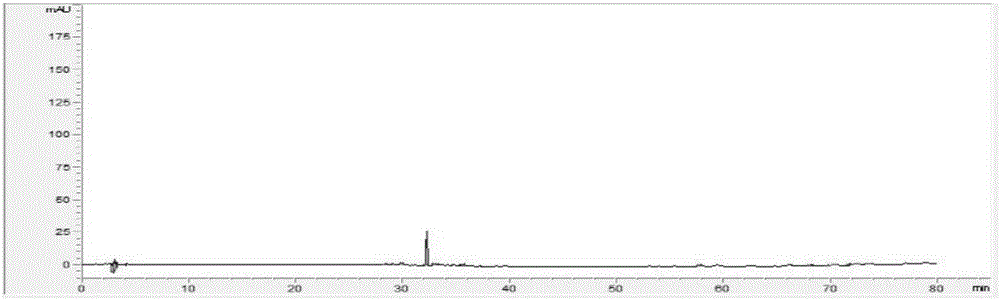
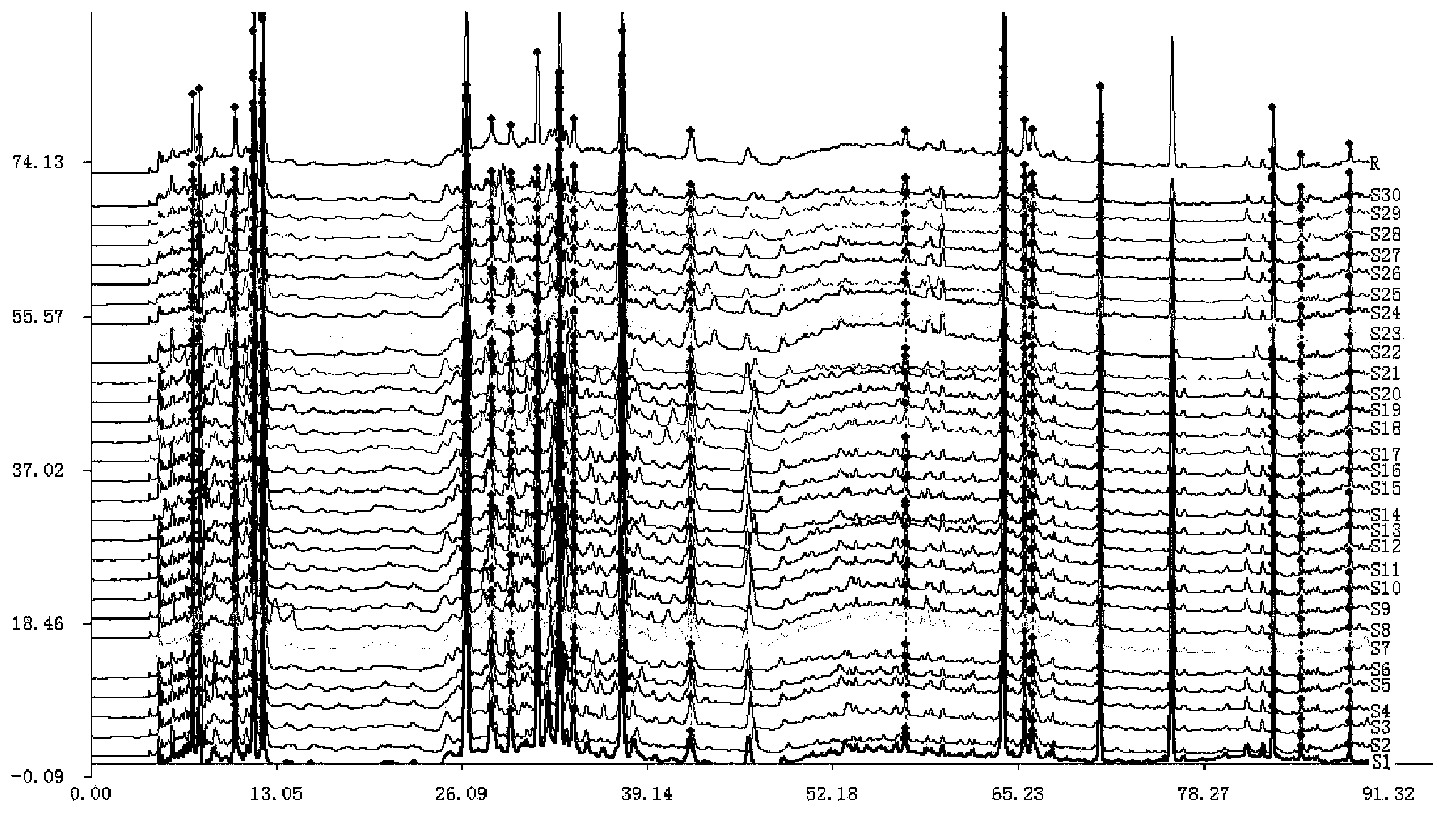
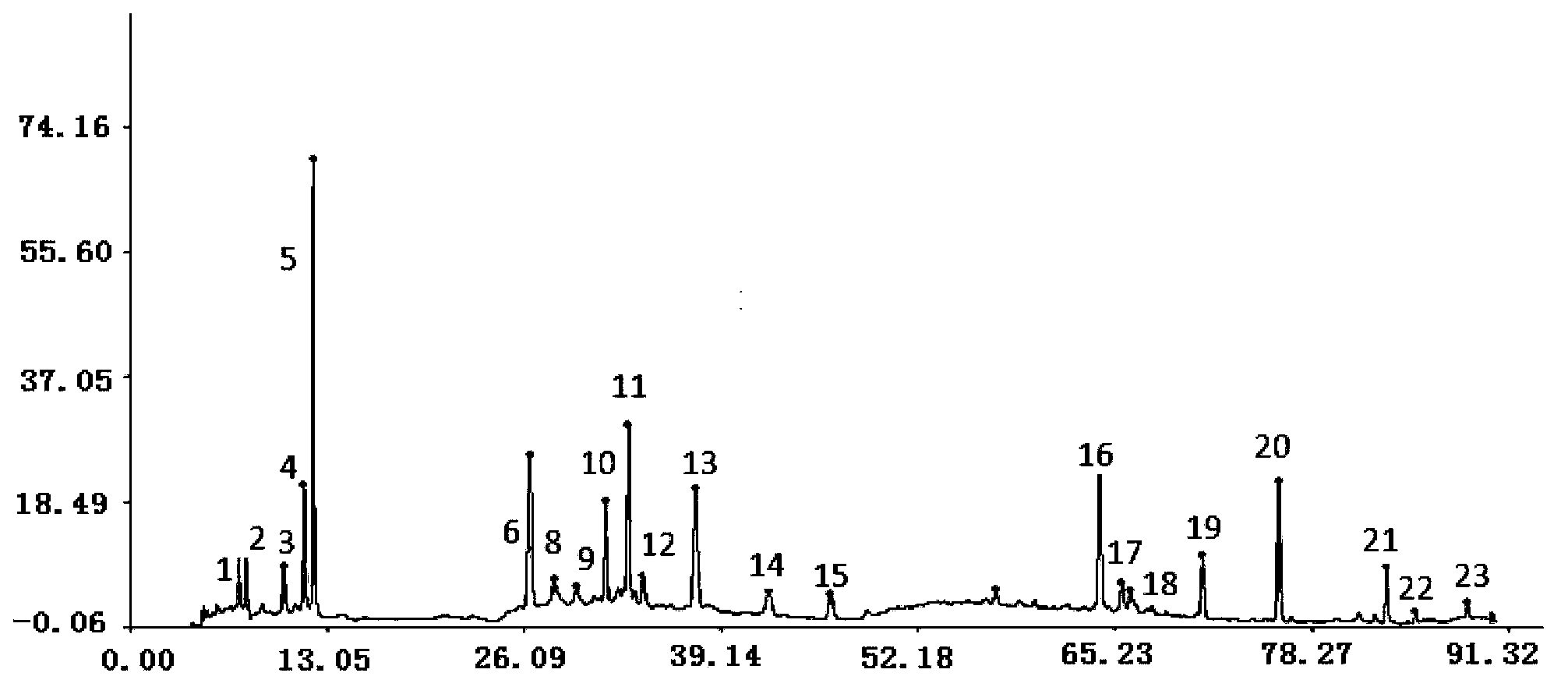
Measurement method of radix astragali granule fingerprint and characteristic fingerprint thereof
ActiveCN103149300BScientific Evaluation QualityHigh technical contentComponent separationMedicinePeak area
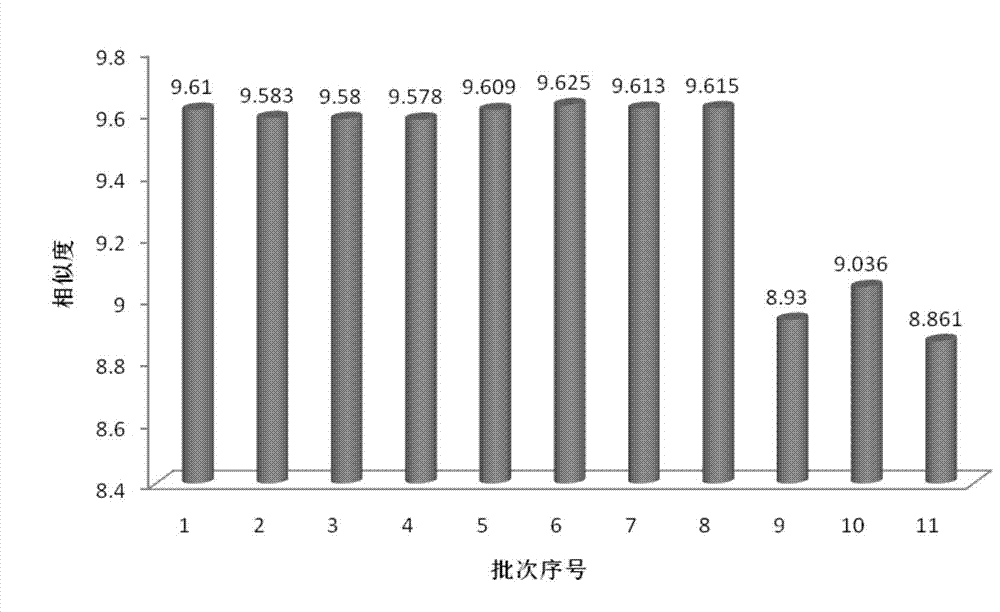
The invention discloses a measurement method of a radix astragali granule fingerprint. The measurement method of the radix astragali granule fingerprint comprises the steps of preparation of a test solution, preparation of a comparison product solution, high performance liquid chromatograph measurement, and treatment of data and fingerprint. The invention further discloses the radix astragali granule fingerprint obtained by the method. Compared with the prior art, the radix astragali granule fingerprint obtained by the invention has the characteristics of more peaks, good peak form, good peak separation effect, larger peak area, easiness of verification, high similarity, accuracy and reliability. The method provided by the invention is simple and convenient, fast, accurate, stable and reliable, can be used for the quality control of a radix astragali granule sample, can be used for comprehensively, objectively and scientifically evaluating the quality of radix astragali granules, and provides the foundation and the reference for the rational drug use and further drug efficacy study of the radix astragali granules.
Owner:贵州汉方制药有限公司 +1
Construction method of hplc characteristic map of a kind of Chinese patent medicine "Qingyi Lidan Granule"
ActiveCN108362790BAvoid monotonyAvoid one-sidednessComponent separationPaeonia albifloraTheoretical plate
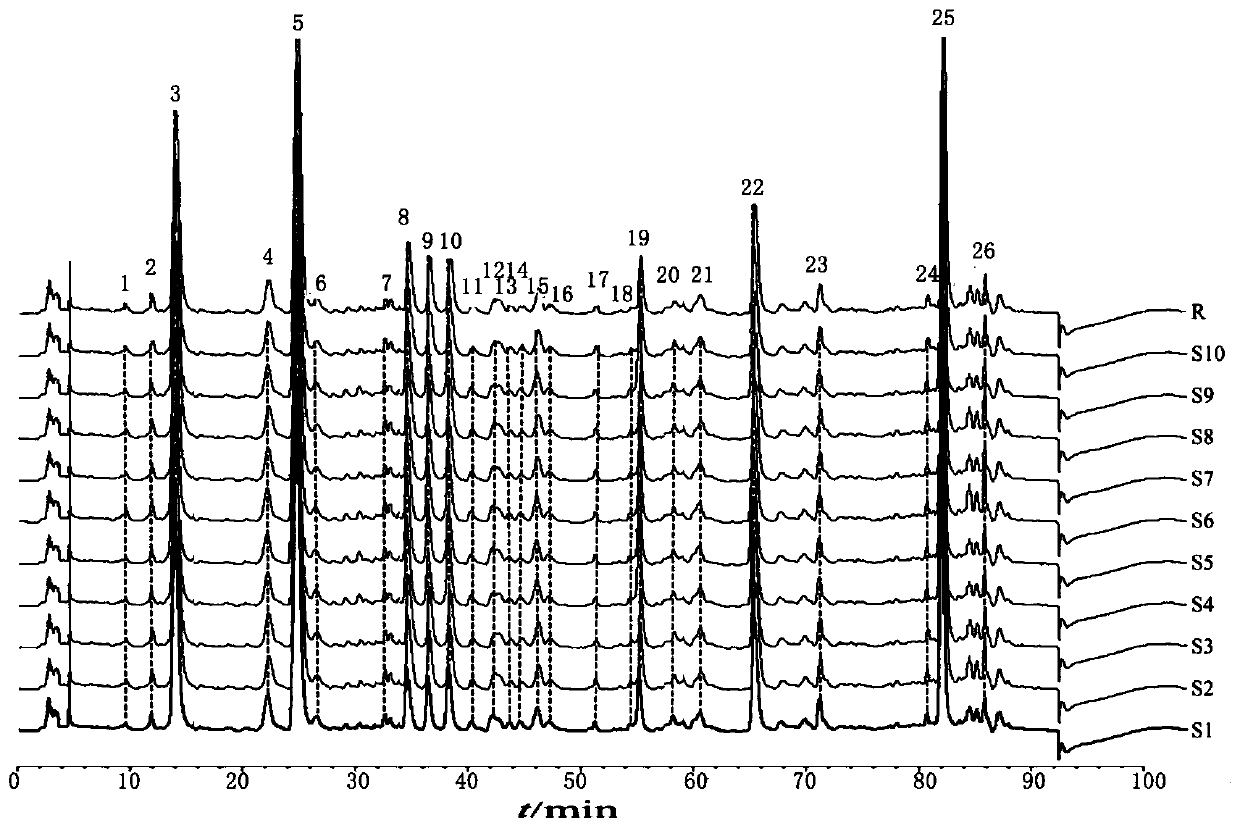
A method for constructing a HPLC characteristic spectrum of a Chinese patent medicine "Qingyi Lidan Granule" belongs to the technical field of traditional Chinese medicine, and the method comprises: (1) preparation of a test solution; (2) preparation of a reference solution; (3) chromatography Conditions: Agilent ZORBAX SB‑C18 (5μm, 4.6×250mm) column, mobile phase A is acetonitrile, mobile phase B is 0.2% phosphoric acid solution, gradient elution, flow rate 0.9~1.1ml / min, column temperature 30~ 40℃, the detection wavelength is 235~245nm. The theoretical plate number should not be less than 3000 according to the paeoniflorin peak. (4) Determination: Determination of characteristic spectrum according to high performance liquid chromatography. By establishing the HPLC characteristic chromatogram of 10 batches of Qingyi Lidan granules, 10 characteristic peaks were determined, and the standard characteristic chromatogram of Qingyi Lidan granules was established. The invention has the characteristics of convenience, speed, stability, high precision, good reproducibility and the like, and can effectively control the quality of the Qingyilidan granules.
Owner:抚松县中药有限责任公司
Detection method of hplc fingerprint of Radix Polygoni Multiflori
The invention discloses a raw fleece-flower root HPLC fingerprint detection method. The detection method comprises the following steps: (1) preparing a control substance solution; (2) preparing a reference substance solution; (3) establishing a control fingerprint; (4) preparing a test article solution; (5) detecting the test article solution. The obtained fingerprint has the advantages that there are many peaks, the resolution and repeatability are good, the precision is high, and the quality of raw fleece-flower root can be effectively monitored. By using the extraction method and chromatogram conditions of provided fingerprint detection method, contents of 16 components of raw fleece-flower root can be measured, and thus the quality of raw fleece-flower root can be monitored from more aspects.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Loquat lung-heat-clearing beverage contrast extract, preparation method and quality control method
ActiveCN114689766AReduce consumptionImprove creativityComponent separationAgainst vector-borne diseasesBiotechnologyMedicinal herbs
The invention discloses a loquat lung-heat-clearing drink reference extract, a preparation method and a quality control method, and belongs to the technical field of traditional Chinese medicine detection.The loquat lung-heat-clearing drink reference extract freeze-dried powder stable in quality is obtained by controlling technological parameters of medicinal material pretreatment, decoction, concentration, freeze drying and the like of a loquat lung-heat-clearing drink prescription. The freeze-dried powder is used for quality research of the loquat lung-heat-clearing drink, the quality is uniform and stable, the detection result is reliable, the specific chromatogram and the identification method are suitable for qualitative detection of various dosage forms of the loquat lung-heat-clearing drink, the related specific chromatogram construction method discloses a detection method of a reference extract of the loquat lung-heat-clearing drink with raw materials easy to obtain, and the method is suitable for large-scale popularization and application. The detection method is simple, convenient and reliable, and provides powerful guarantee for qualitative identification of the loquat lung-heat-clearing drink and the preparation thereof.
Owner:SICHUAN NEO GREEN PHARMA TECH DEV
Method for constructing HPLC (high performance liquid chromatography) fingerprint spectrum of medicinal leaves and twigs of rhododendron mariae
ActiveCN110274976AMonitor qualityAvoid monotonyComponent separationHplc fingerprintRhododendron mariae
The invention provides a method for constructing the HPLC (high performance liquid chromatography) fingerprint spectrum of the medicinal leaves and twigs of rhododendron mariae. The method comprises the following steps of: (1) test solution predation: the leaves and twigs of the rhododendron mariae are crushed and weighed, an obtained substance is arranged in an extractor, petroleum ether is added to the extractor, heat reflux extraction is carried out, and the petroleum ether is discarded, and methanol is added, heating reflux is carried out, methanol is supplemented, and filtering is carried out; (2) reference substance solution preparation: rutin, quercitrin, hyperoside, quercetin, kaempferol, an oleanolic acid reference substance are selected, and a mixed reference substance solution is prepared with methanol adopted as a solvent; and (3) and measurement: the reference substance solution and the test solution are injected into a liquid chromatograph separately, and chromatograms are recorded in 105 minutes, and the chromatograms are processed with fingerprint spectrum software, so that the fingerprint spectrum of the leaves and twigs of the rhododendron mariae can be obtained. With the method for constructing the HPLC (high performance liquid chromatography) fingerprint spectrum of the medicinal leaves and twigs of the rhododendron mariae of the invention adopted, the common model of the HPLC (high performance liquid chromatography) fingerprint spectrum of the medicinal leaves and twigs of the rhododendron mariae is established, and 16 common peaks are calibrated, so that the quality of the medicinal leaves and twigs of the rhododendron mariae can be effectively characterized; the singleness and one-sidedness of an original quality control method are avoided. The method has a relatively high application value.
Owner:CHANGCHUN UNIV OF CHINESE MEDICINE
Determination method of chemical components in Chinese herbaceous peony and liquorice decoction and establishment method of fingerprint spectrum
PendingCN114152683AEasy to separateEasy to identifyComponent separationBiotechnologyBULK ACTIVE INGREDIENT
The invention belongs to the field of detection, and provides a method for determining chemical components in paeonia lactiflora and liquorice decoction and a method for establishing a fingerprint spectrum, the method comprises the following steps: mixing a paeonia lactiflora and liquorice decoction sample with a solvent, and dissolving to obtain a to-be-detected solution; the method comprises the following steps: weighing a reference substance, respectively obtaining chromatograms of chemical components in a paeonia lactiflora and liquorice decoction solution and the reference substance under the same detection conditions by adopting a high performance liquid chromatography, and determining the content of the paeonia lactiflora and liquorice decoction according to the concentration of the reference substance, the peak area of the reference substance in the chromatograms and the peak area of components corresponding to the reference substance in the paeonia lactiflora and liquorice decoction in the chromatograms. And calculating the contents of active ingredients of paeoniflorin, liquiritin and ammonium glycyrrhizinate in the Chinese herbaceous peony and liquorice decoction by an external standard two-point method based on a dual-wavelength detection condition. According to the method, under the same chromatographic condition, the chemical components in the paeonia lactiflora and liquorice decoction and the specific content of the corresponding chemical components can be simultaneously identified and detected only by adjusting the wavelength, the result is more accurate and reliable, and good reproducibility and stability are achieved.
Owner:CHENGDU HUASUN GRP INC LTD
Establishment method of lindley eupatorium herb formula granule fingerprint as well as standard fingerprint and application thereof
ActiveCN114216978AFully reflect the quality statusFully extractedComponent separationMedicinal herbsPhysical chemistry
The invention provides a construction method of a lindley eupatorium herb formula granule fingerprint spectrum, a standard fingerprint spectrum of the lindley eupatorium herb formula granule and application of the standard fingerprint spectrum, and belongs to the technical field of medicine detection.The construction method comprises the steps that a test solution, a reference medicinal material solution and a reference substance solution are prepared, acetonitrile (A) and a 0.1-0.3 wt% phosphoric acid aqueous solution (B) are jointly used as mobile phases, and the standard fingerprint spectrum of the lindley eupatorium herb formula granule is obtained. Performing high performance liquid chromatography detection to obtain a fingerprint spectrum of the lindley eupatorium herb formula granules; the standard fingerprint spectrum of the lindley eupatorium herb formula granules is obtained by using the construction method, and the standard fingerprint spectrum is used for quality evaluation or control in the whole process of research / development / production / clinical application of the lindley eupatorium herb formula granules. According to the method for constructing the fingerprint spectrum of the lindley eupatorium herb formula granules, main medicinal material components in the lindley eupatorium herb formula granules can be detected only through high performance liquid chromatography, and the quality condition of the lindley eupatorium herb formula granules can be comprehensively reflected.
Owner:SHINEWAY PHARMA GRP LTD +2
A method for constructing hplc fingerprint of Xiaoerfeike granule
ActiveCN113237974BComprehensive chemical informationCharacterizing qualityComponent separationHplc fingerprintMedicinal herbs
The invention relates to a method for constructing an HPLC fingerprint of Xiaoerfeike granules, which belongs to the field of medicine. According to the HPLC fingerprint of Xiaoerfeike granules, the invention optimizes the conditions of the mobile phase, detection wavelength, gradient elution procedure and the like, and establishes the detection conditions of the fingerprint. Based on multiple batches of samples, a standard fingerprint of Xiaoerfeike Granules was established, which identified 24 common peaks, and carried out the identification of medicinal materials and components of the related common peaks. The method of the invention can comprehensively control the quality of Xiaoer Feike Granules, so as to better ensure the quality stability, consistency and controllability of Xiaoer Feike Granules, thereby ensuring the safety and effectiveness of Xiaoer Feike Granules . The present invention comprehensively analyzes the status of each component in Xiaoer Feike Granules through systematic identification of components and the attribution of single medicinal materials with common peaks in different batches of samples, which can provide an important reference for the quality control of Xiaoer Feike Granules.
Owner:CHANGCHUN RENMIN PHARMA GROUP
A kind of fingerprint detection method of Wenjing Decoction
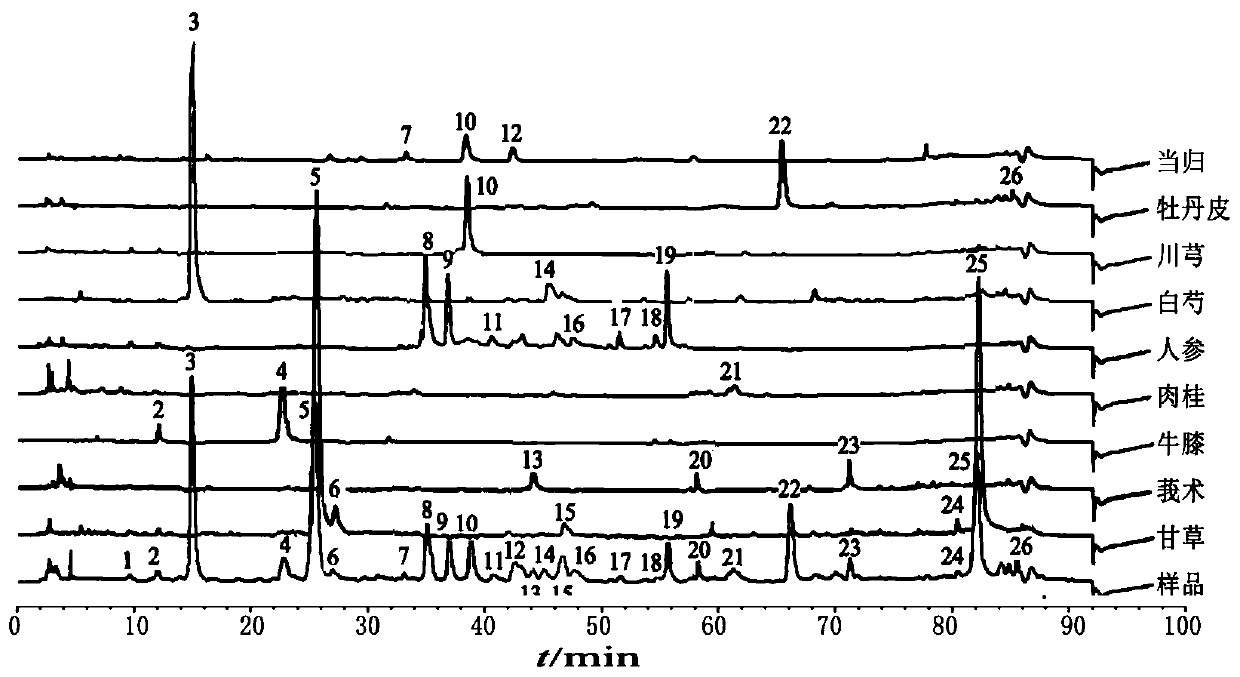
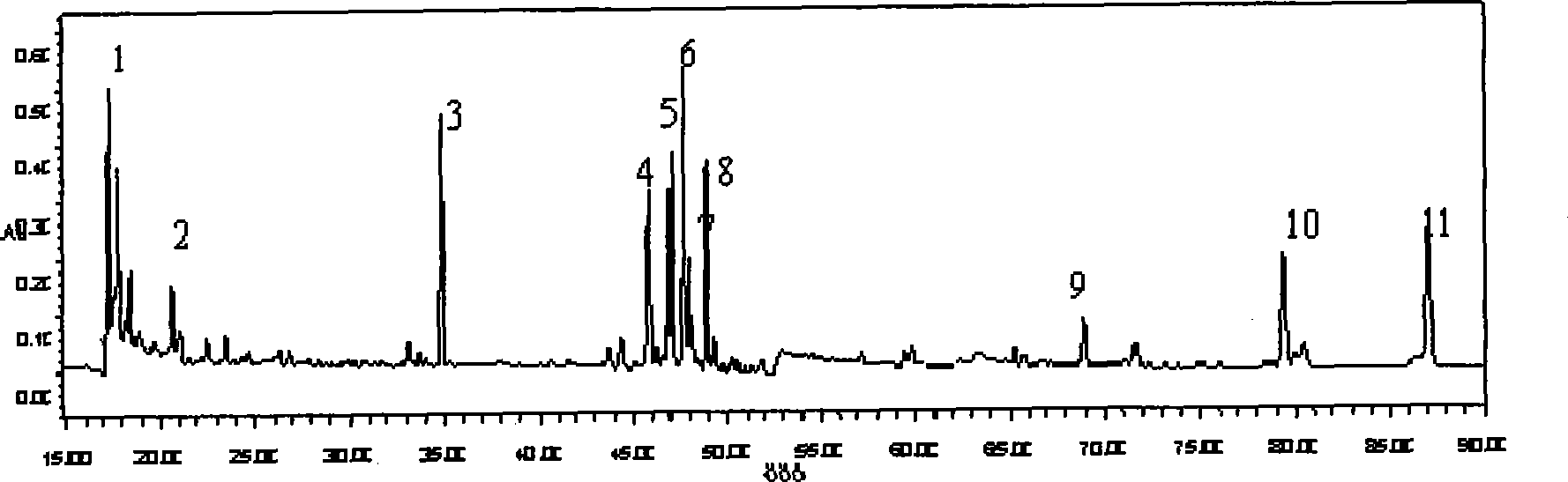
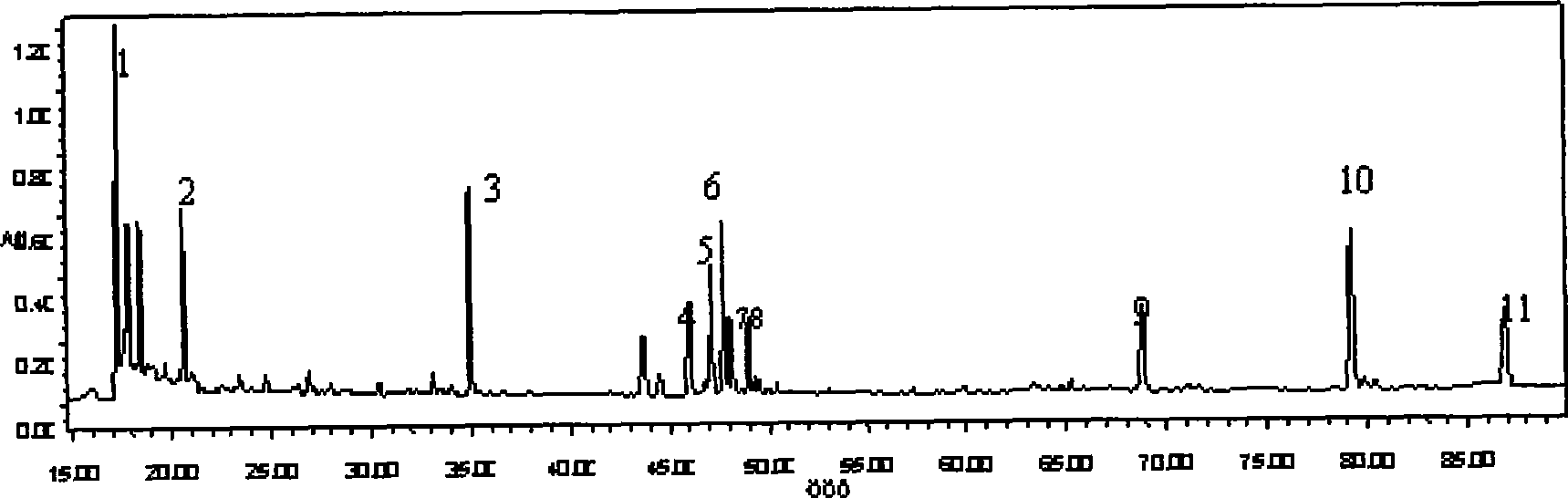
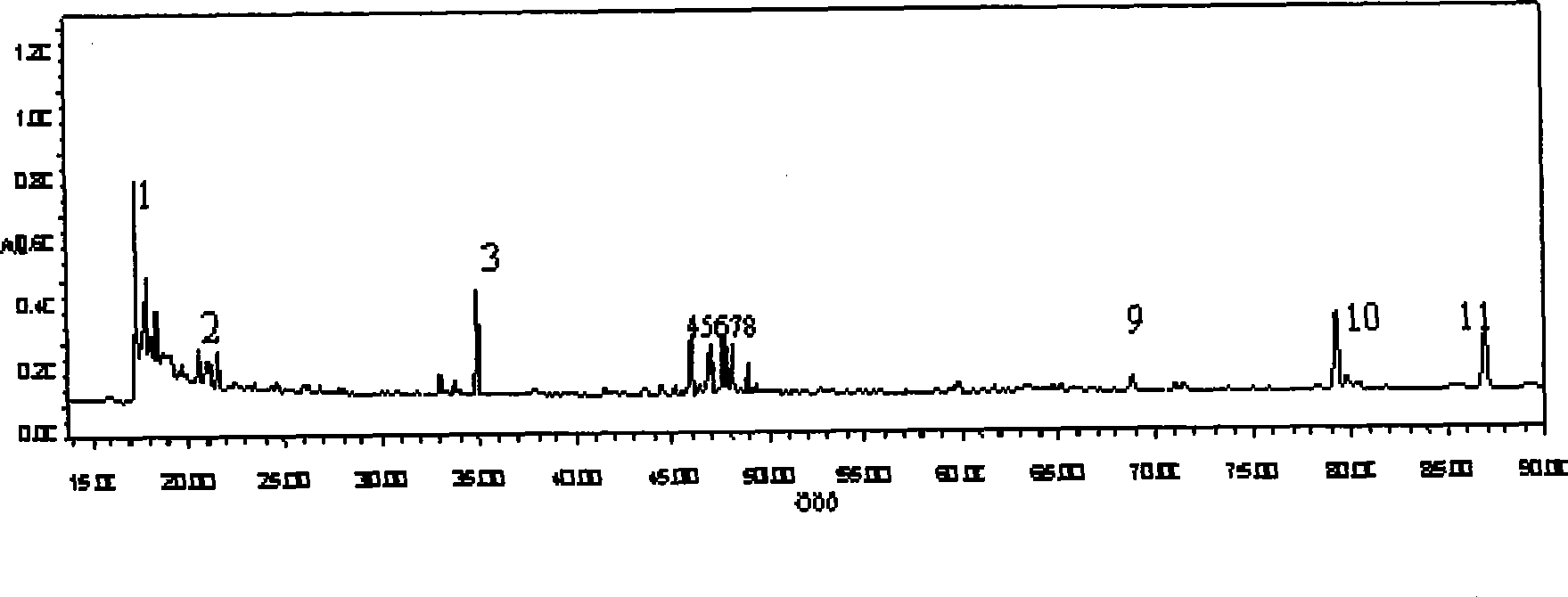
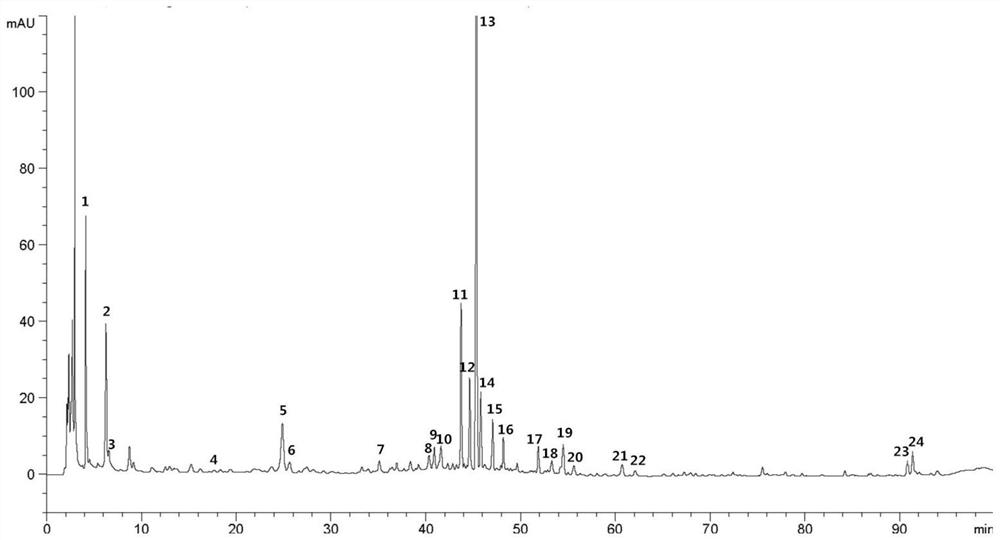
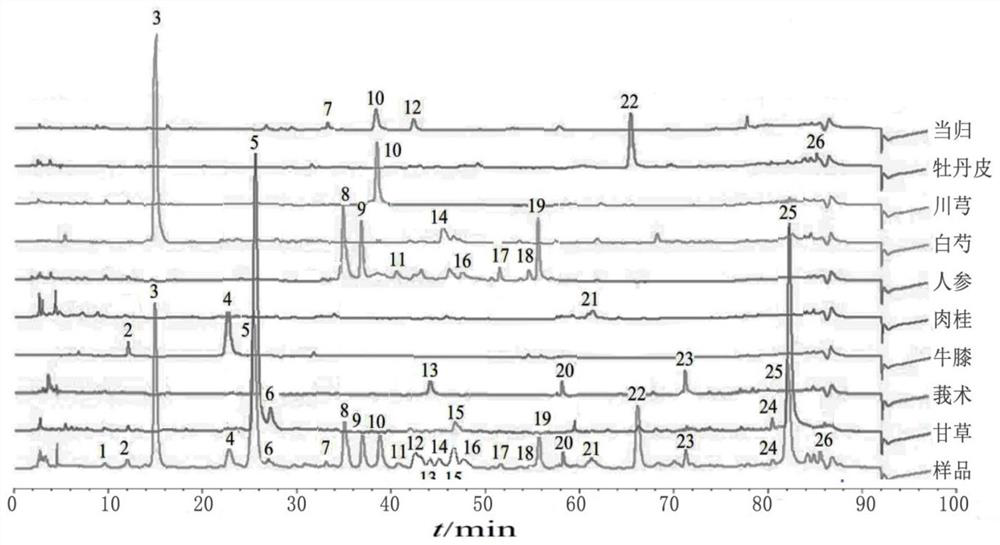
ActiveCN110907553BImprove stabilityGood reproducibilityComponent separationBiotechnologyAngelica Sinensis Root
The present invention relates to a kind of fingerprint detection method of Wenjing Decoction, described method, the steps are as follows: 1) preparation of test solution: take by weighing 1-2g each of Angelica sinensis, Rhizoma Chuanxiong, Radix Paeoniae Alba, Cinnamon, Cortex Moutan, and Curcuma officinalis powder, Ginseng, licorice, and Achyranthes bidentata powder are 3-4g each, add 250-350mL of water, mix well, first heat to boiling with strong fire, then switch to slow fire and fry until the decoction reaches about 150-170mL, filter while hot, and the filtrate Add water to make up to 240‑260mL; accurately draw 8‑12mL of Wenjingtang standard decoction, add methanol to make up to 40‑60mL, airtight, weigh, ultrasonicate for 8‑12min, leave overnight, weigh again, use methanol Make up the lost quality, shake well, filter, and get the filtrate to get final product; 2) detection: take 5-15 μL of the test solution obtained in the previous step, inject it into a high-performance liquid chromatograph, and obtain a chromatogram; 3) result Judgment: The chromatogram obtained in the previous step is compared with the standard control fingerprint. If the similarity is greater than 90%, the sample is qualified.
Owner:SHANDONG WOHUA PHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com