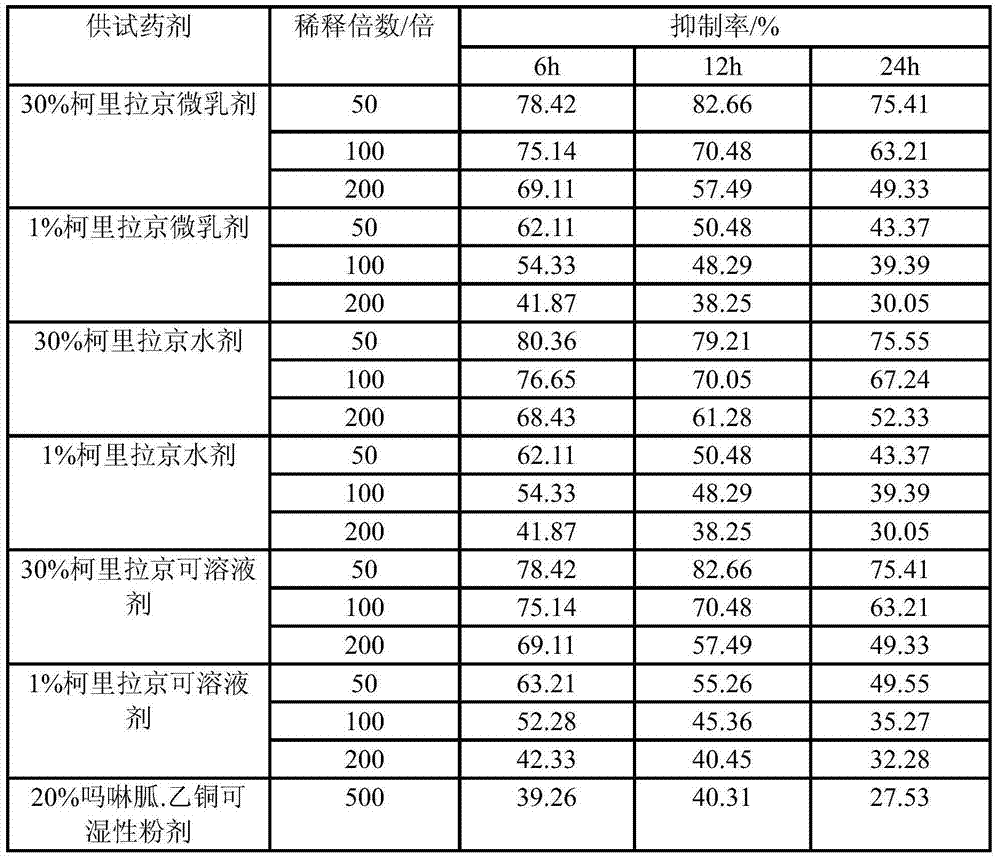
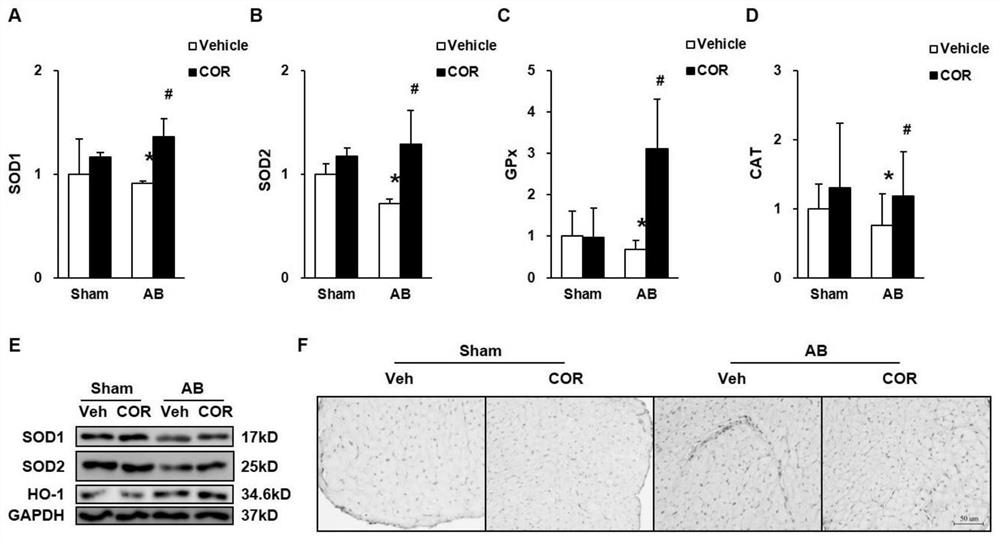
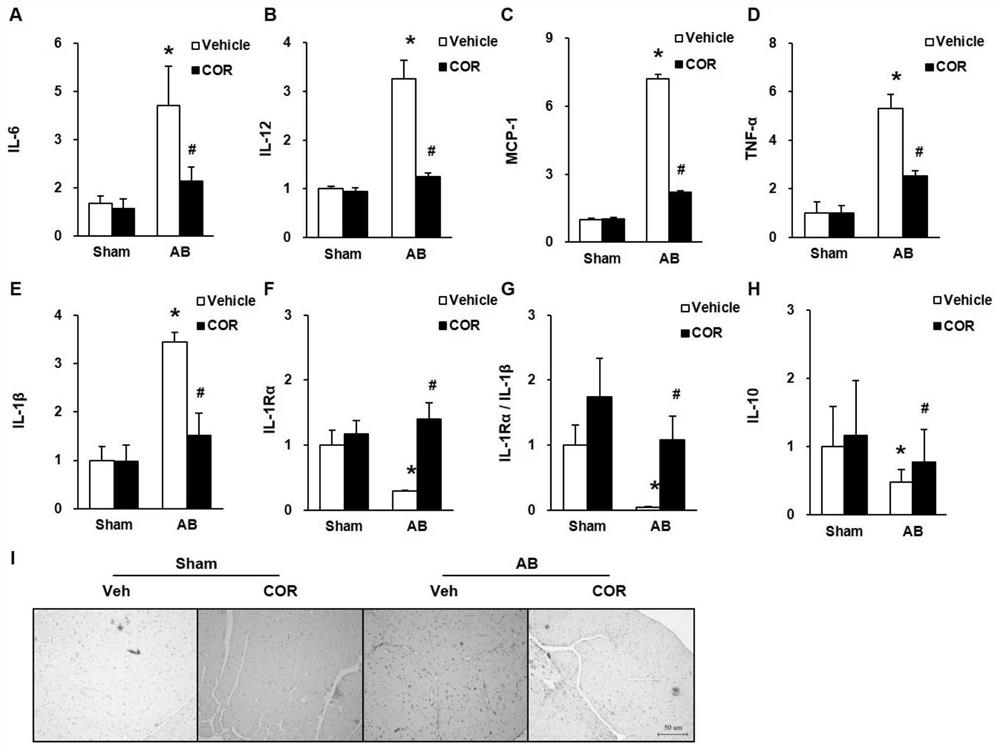
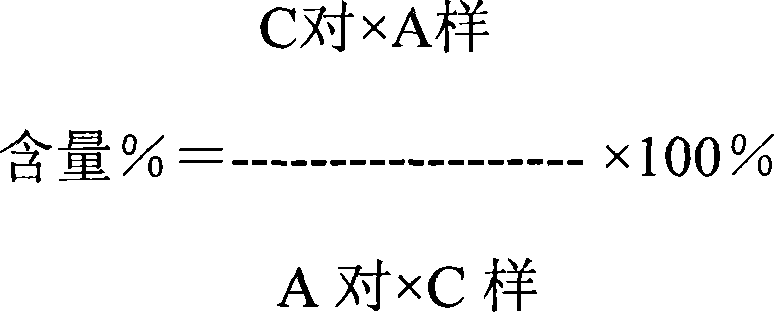
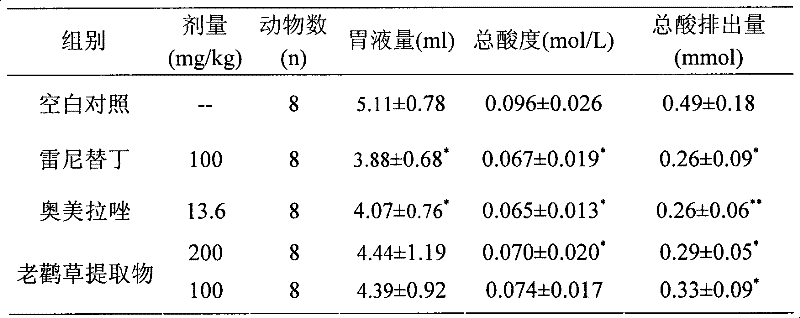
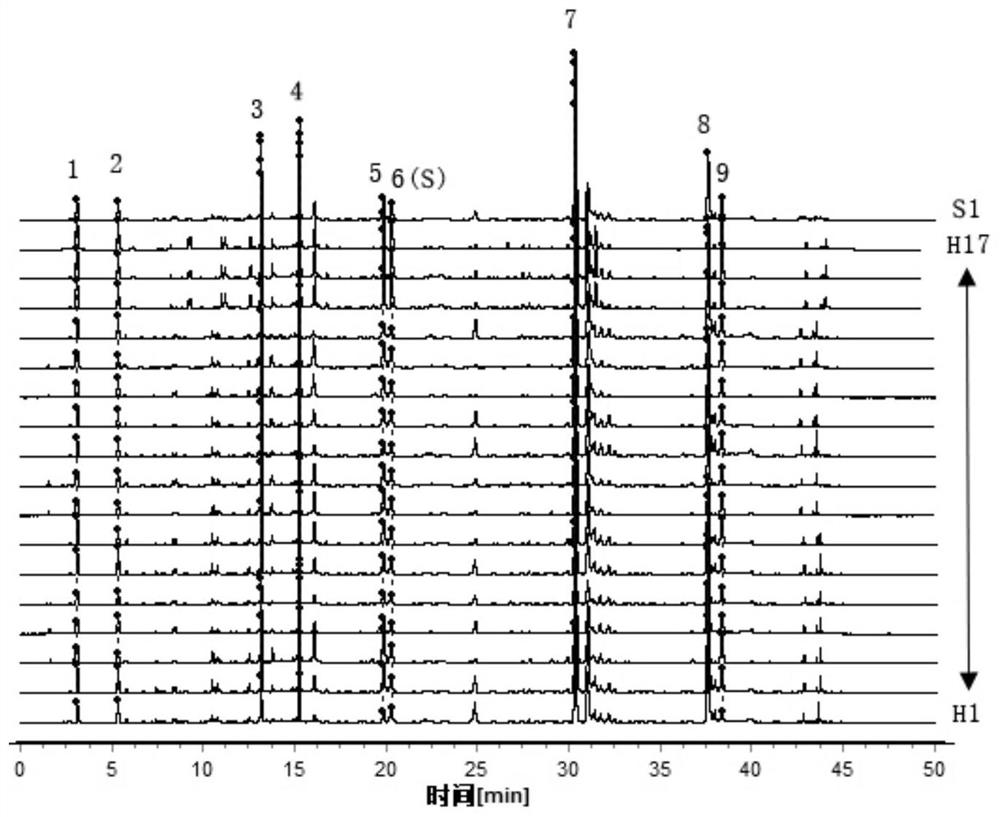
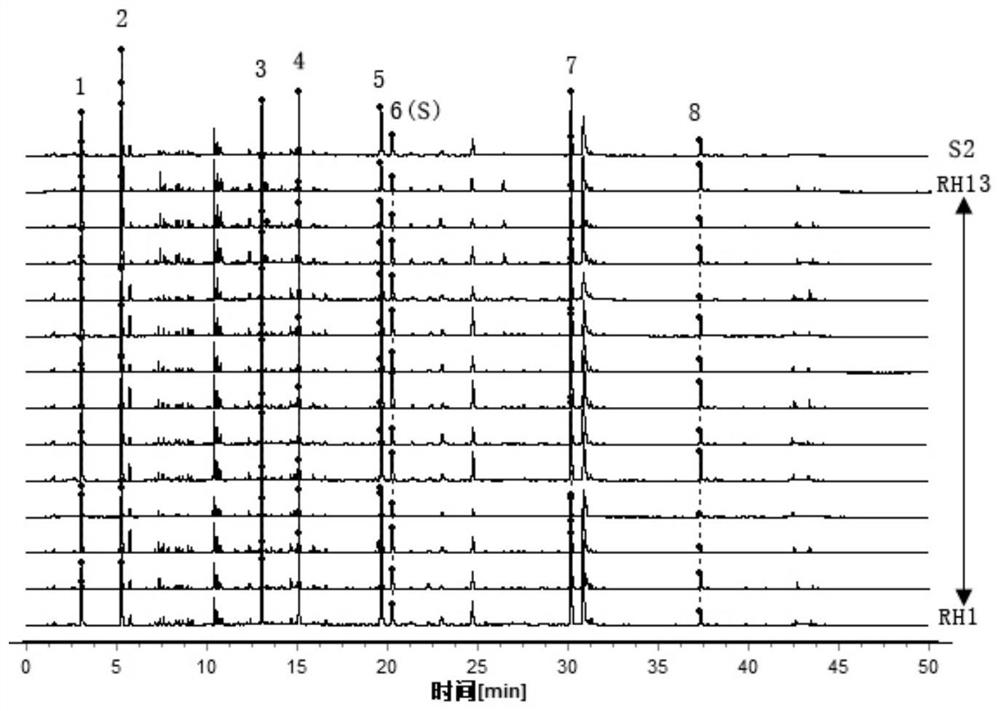
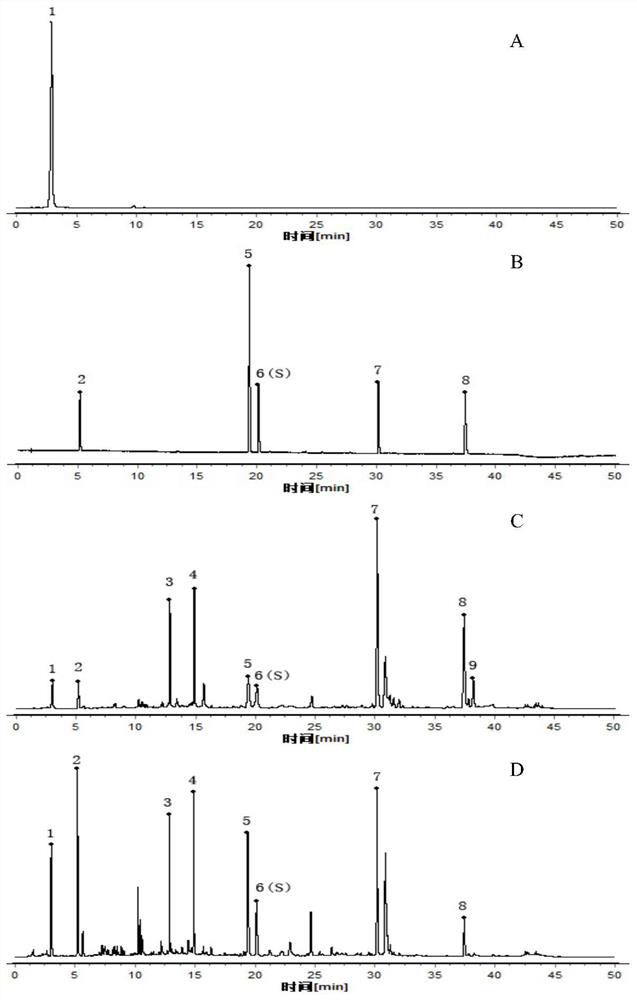
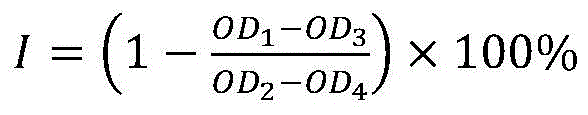Patents
Literature
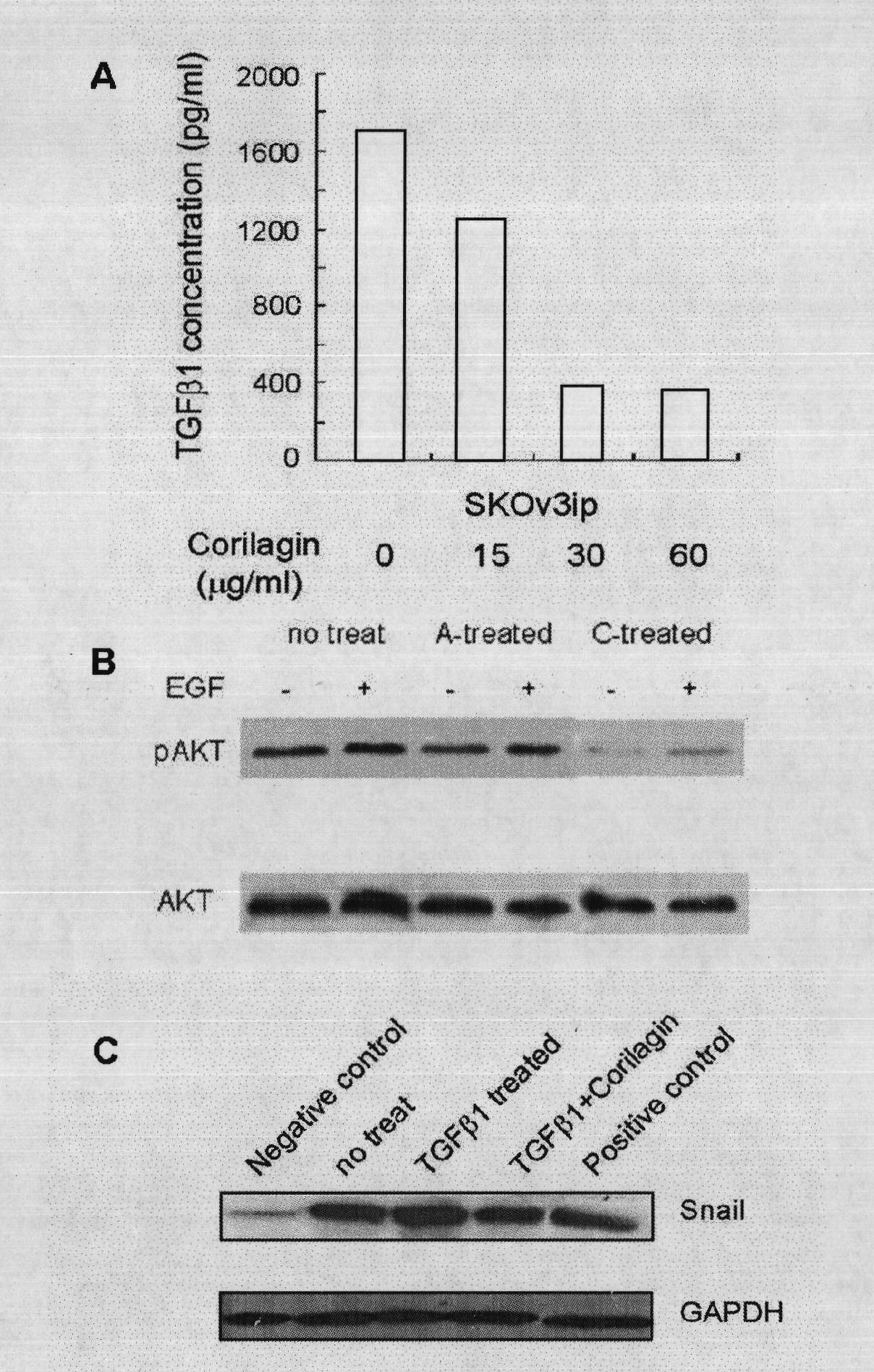
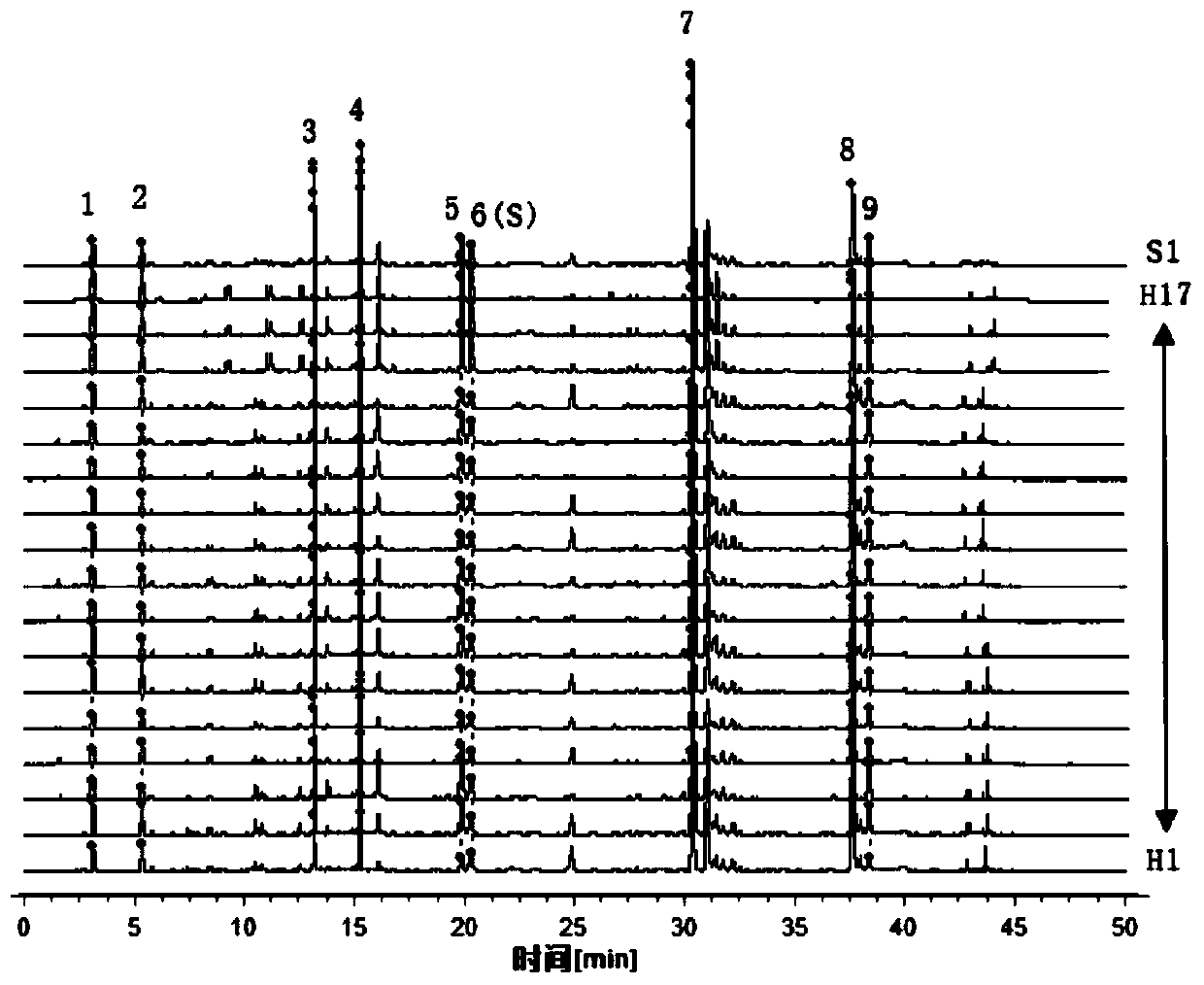
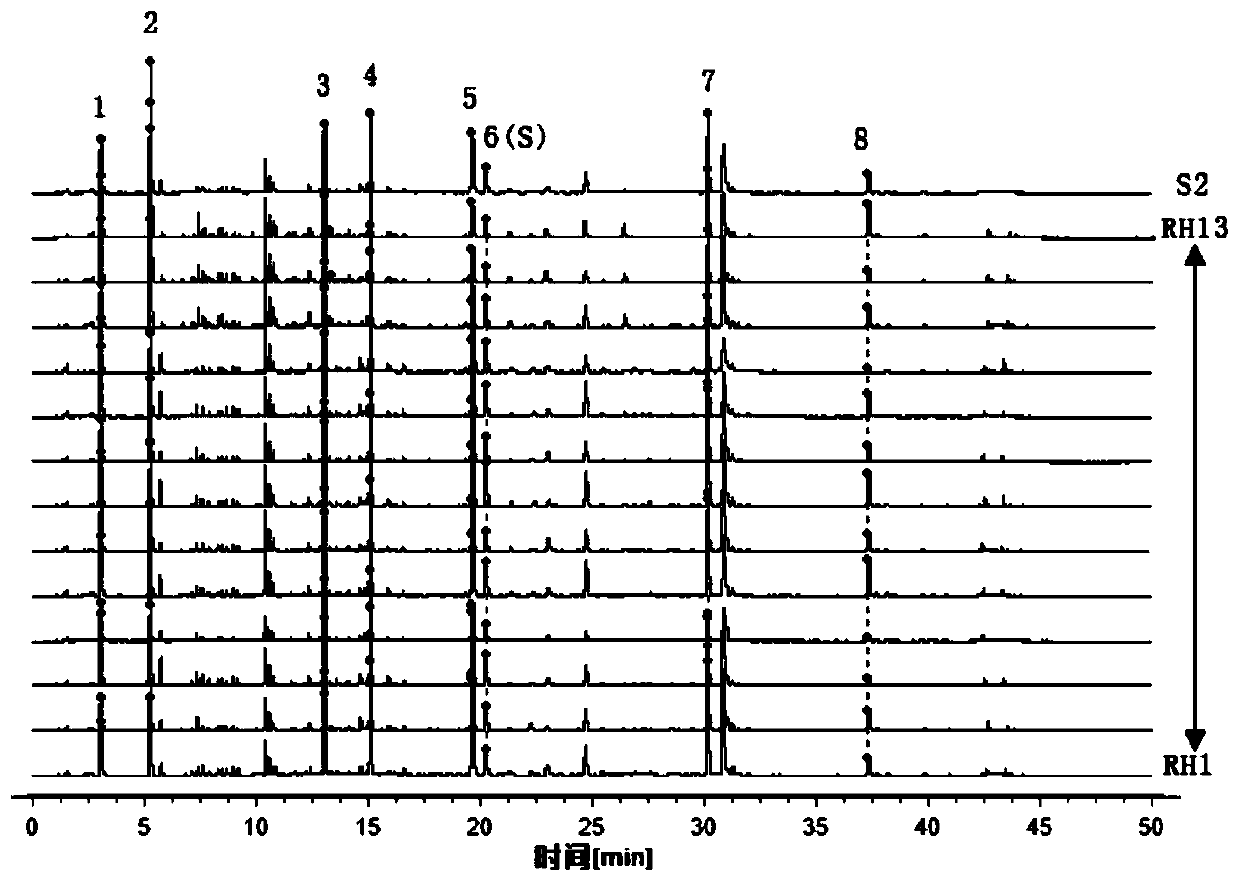
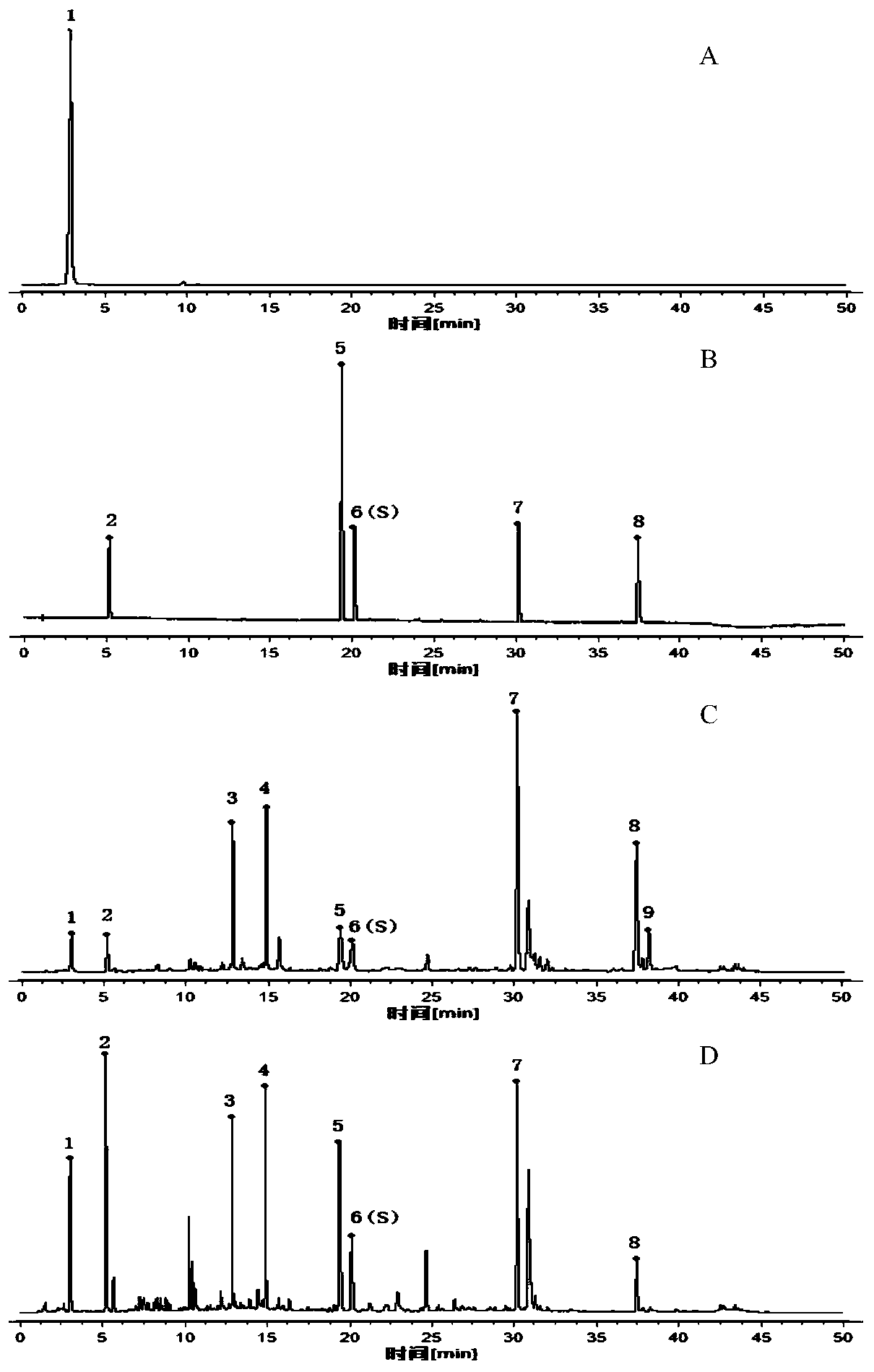
32 results about "Corilagin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
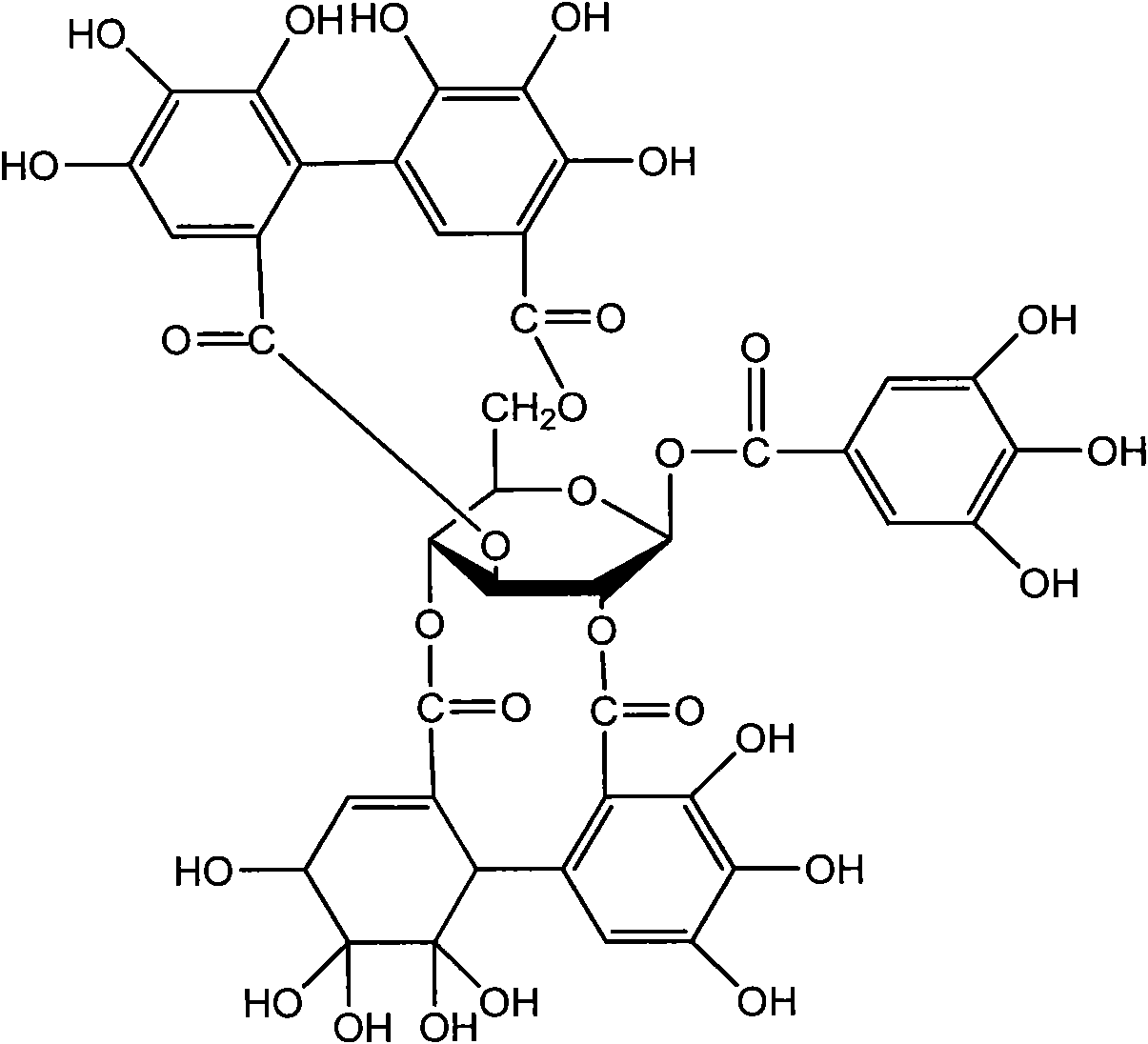
Corilagin is an ellagitannin. Corilagin was first isolated in 1951 from Dividivi extract and from Caesalpinia coriaria, hence the name of the molecule. It can also be found in Alchornea glandulosa and in the leaves of Punica granatum (pomegranate).
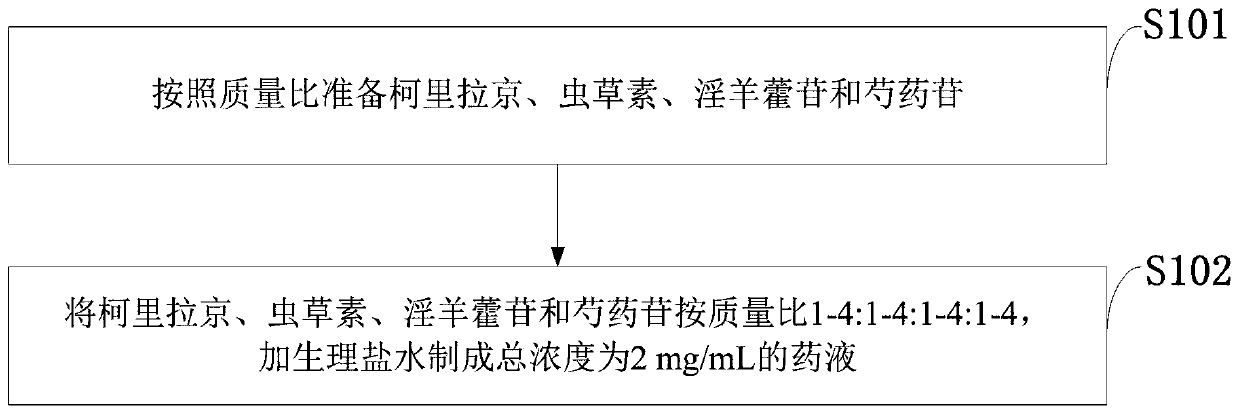
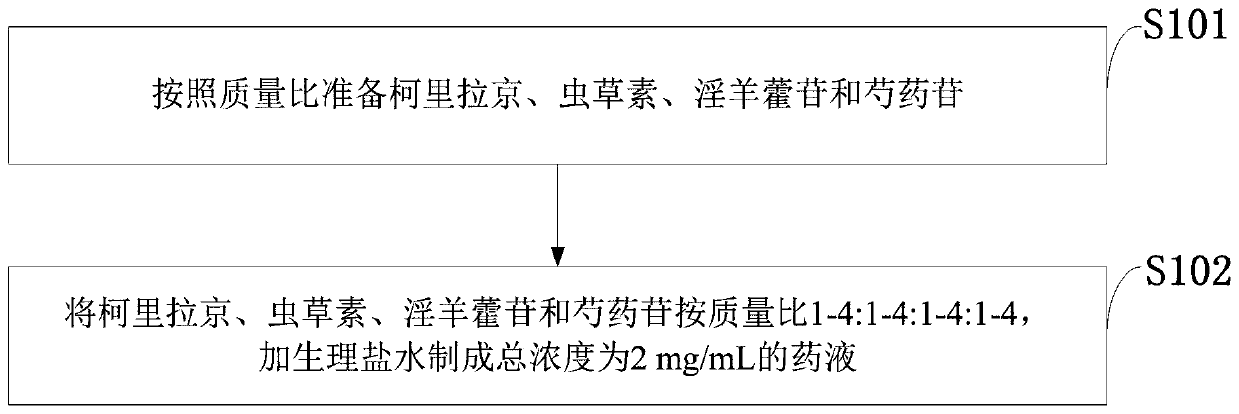
A method for extracting and purifying corilagin
InactiveCN102286031AHigh extraction rateHigh purityEsterified saccharide compoundsSugar derivativesState of artDesorption
The invention relates to a method for extracting and purifying corilagin, which comprises: absorbing and analyzing the extract of Phyllanthus phylloxera through macroporous resin, concentrating the desorption liquid and extracting it with n-butanol, and then extracting the extracted liquid with supercritical carbon dioxide fluid to obtain corylatin. Lilatine extract, the extract is further purified by gel column chromatography, and recrystallized to obtain Corilagin. Compared with the prior art, the preparation method has simple operation, low cost and is suitable for industrial production.
Owner:NANJING ZELANG MEDICAL TECH
Application of polyphenols in resisting coronavirus
The invention discloses an application of various compounds such as polyphenols in resisting coronavirus. The invention relates to the application of one or more of a polyphenol substance, a proton pump inhibitor, p-benzoquinone and a derivative thereof, an active ingredient of sappan wood, TDZD-8, thiomersalate, alkannin, Tidelusib, protoflavin, rabeprazole sodium, PX-12, Dixanthophygen, methyl cholate, carbamofluorine, corilagin, dihydromyricetin, chloramine T, merbromin, gallocatechin, fraxetin, meisoindigo, a lactic acid ethacridine monohydrate and sousoprazole in preparation of drugs for treating and / or preventing diseases caused by coronavirus. In-vitro enzyme activity experiments show that various compounds such as 1, 4-naphthoquinone and the like can well inhibit the activity of main protease in the coronavirus, and the defect that diseases caused by the coronavirus cannot be treated in the prior art is overcome.
Owner:SHANGHAI TECH UNIV
Geranium extract
InactiveCN101461831ASimple preparation processReduce secretionSugar derivativesDigestive systemDiseaseMedicine
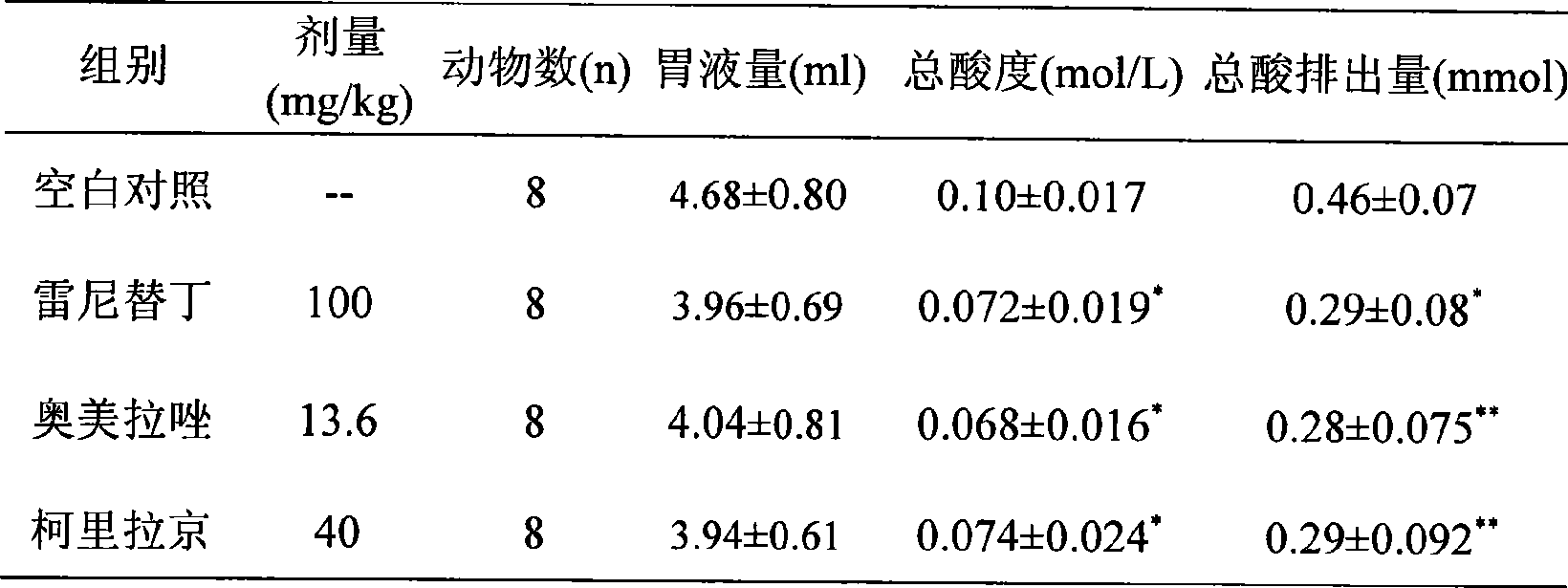
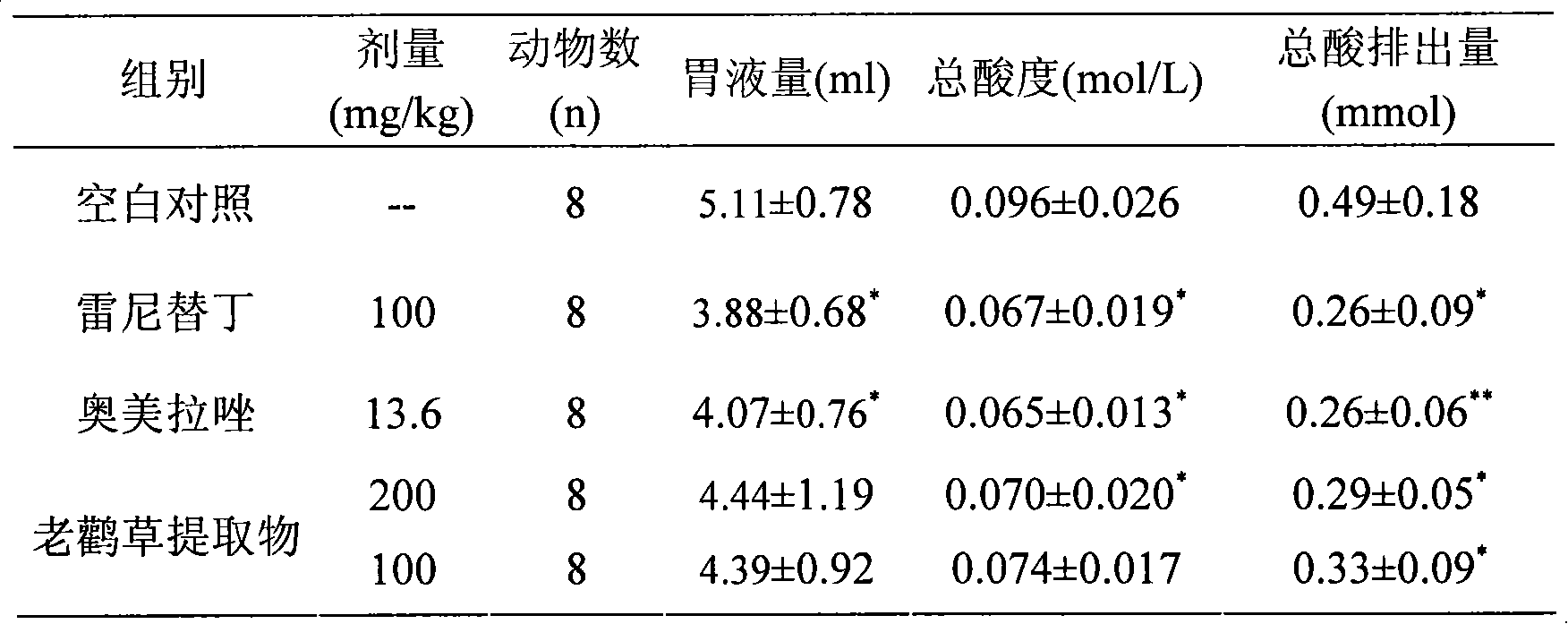
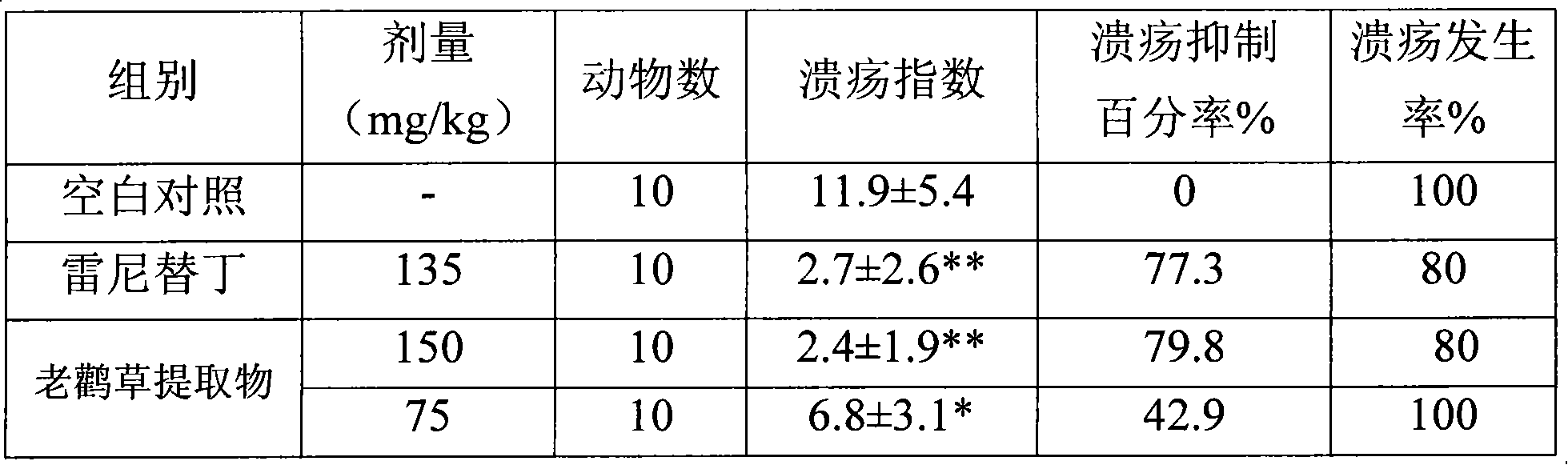
The invention relates to a novel heroubill geranium extract and a preparation method thereof, in particular to a heroubill geranium extract containing corilagin and application of the heroubill geranium extract in medicines for treating or preventing diseases related to gastric acid hypersecretion.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for extracting polyphenol active ingredient in geranium wilfordii through high-efficiency enzyme induction
InactiveCN101849978AOperational securityRealize industrial productionAntibacterial agentsDigestive systemGallic acid esterCavitation
The invention relates to an extraction technology of active ingredients in geranium wilfordii which is one Chinese medicinal herb. The purpose of the invention is to provide an economic and rapid method for extracting polyphenol active ingredients in geranium wilfordii through high-efficiency enzyme induction. The invention adopts a homogenizer fragmentation high-efficiency enzymatic hydrolysis technology and combines a negative-pressure cavitation assisted extraction technology to obtain geranium wilfordii extract rich in three kinds of polyphenol active ingredients. According to results, the maximum content of total polyphenol active ingredients in the extract can reach 156.14mgGAE / g, the maximum contents of three main polyphenol active ingredients, i.e. gallic acid, Corilagin, Geraniin can respectively reach 9.58mg / g, 6.87mg / g and 19.82mg / g, and the contents are improved by 30-67 percent when compared with a traditional hot reflux method. The invention has the advantages that the high-temperature heating is not required, the organic solvent is not required, the extraction ratio is high, the environment is protected, the operation is safe, the method is suitable for industrialized production, and a novel method and a novel means are provided for the modernization of Chinese medicine extraction.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Extraction-separation method and application of corilagin
InactiveCN104447896AEasy to recycleReduce oil soluble componentsEsterified saccharide compoundsCosmetic preparationsAqueous acetonePolyamide
The invention discloses an extraction-separation method and an application of corilagin. The method comprises the steps of treating longan kernel powder once by using anhydrous acetone and extracting by using acetone aqueous solution again for the second time; uniformly mixing a proper amount of chromatography polyamide with the acetone aqueous solution, recycling acetone and applying to a polyamide chromatography column; eluting by sequentially using pure water, methyl alcohol aqueous solutions with different concentrations and methyl alcohol; gathering 40-60 percent of methyl alcohol eluant in a separate bottle form, condensing and separating out corilagin crystals; mixing mother liquid with the rest of eluant containing corilagin, condensing to be dry, dissolving into methyl alcohol and applying to a polydextran gel LH-20 column; eluting by methyl alcohol; gathering eluant in a separate bottle form and standing; mixing with the eluants in which corilagin crystals are separated out; standing again until the orilagin crystals which are separated out are not increased; mixing the obtained corilagin crystals with corilagin crystals obtained by chromatography polyamide; performing recrystallization by using ethyl alcohol to obtain corilagin with high purity. The corilagin subjected to extraction and separation has a function of inhibiting tyrosinase.
Owner:GUANGDONG FOOD & DRUG VOCATIONAL COLLEGE
Novel use of corilagin
InactiveCN101461816AReduce secretionReduce acidityDigestive systemCarbohydrate active ingredientsDiseasePeptic ulcer
The invention relates to novel application of corilagin or a pharmaceutically acceptable corilagin salt, in particular to application of the corilagin or the pharmaceutically acceptable corilagin salt in treating or preventing diseases related to gastric acid hypersecretion such as hyperchlorhydria, peptic ulcer and chronic gastritis.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Emblic leafflower fruit extract capable of dispelling effects of alcohol and protecting liver as well as preparation method and application thereof
InactiveCN111011681AAnti-alcoholic liver protection hasQuench thirstNatural extract food ingredientsFood ingredient as mouthfeel improving agentBiotechnologyGallic acid ester
The invention belongs to the technical field of plant extraction, and particularly relates to an emblic leafflower fruit extract capable of dispelling the effects of alcohol and protecting the liver as well as a preparation method and application thereof. The preparation method comprises the following steps: cleaning fresh emblic leafflower fruits, denucleating and drying the fresh emblic leafflower fruits, crushing the fresh emblic leafflower fruits, carrying out sieving to obtain emblic leafflower fruit micro-powder, adding water, carrying out extracting at 70-90 DEG C for 8-15 minutes, carrying out centrifuging to obtain an emblic leafflower fruit extracting solution, further concentrating the emblic leafflower fruit extracting solution, and adding water for re-dissolving; adding ethylacetate for extraction, and collecting an extraction phase; and further concentrating the extract phase to obtain the emblic leafflower fruit extract capable of dispelling the effects of alcohol and protecting the liver. The main components of the extract are gallic acid, corilagin and ellagic acid, wherein the content of gallic acid is the highest and reaches 2.42mg / g, gallic acid has a good inhibition effect on BRL-3A alcoholic injury, that is, gallic acid has a good protection effect on BRL-3A cells with alcoholic injury, and the damage of alcohol to the liver is further reduced by increasing the SOD content and reducing the MDA content, the ALT content and the AST content.
Owner:GUANGDONG UNIV OF TECH
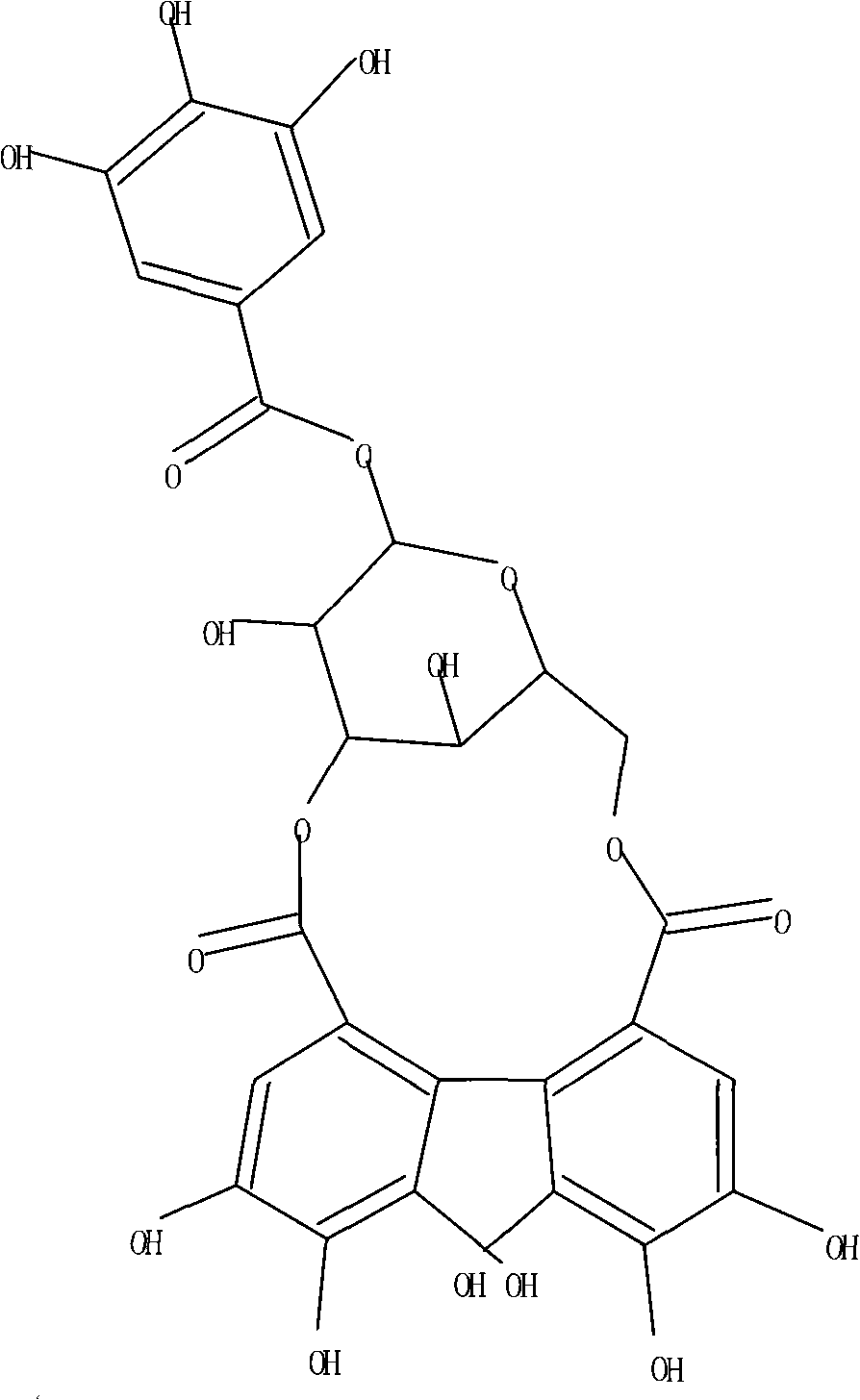
Application of Corilagin in preparing anti-tumor drugs
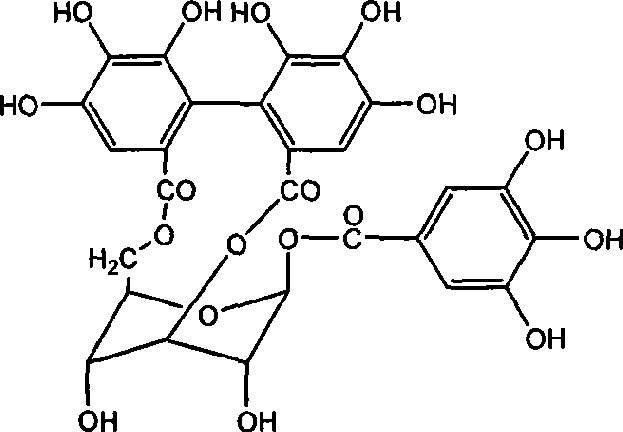
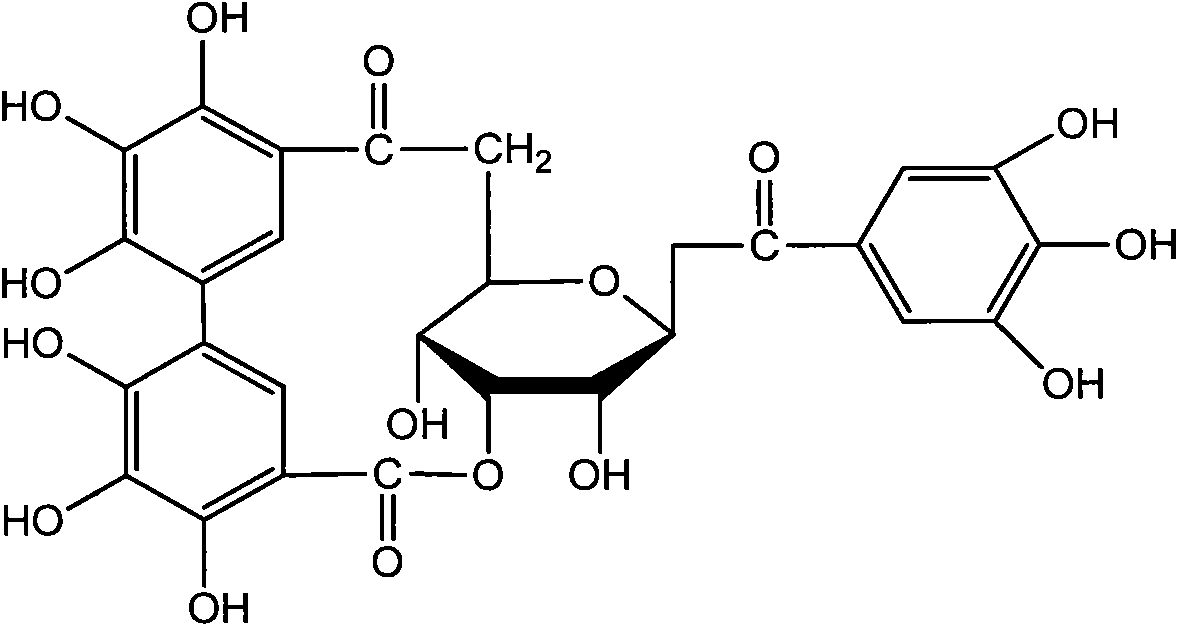
ActiveCN101879173AOrganic active ingredientsAntineoplastic agentsIntraperitoneal routeLymphatic Spread
The invention relates to a Corilagin compound, in particular to the application of Corilagin in preparing anti-tumor drugs. The chemical name of Corilagin is 1-O-trigalloyl-3,6-O-hexahydroxydiphenoyl-beta-D-glucose, and the molecular formula thereof is C27H22O18. The anti-tumor drug for oral administration or injection comprises a drug resisting the tumor growth or a drug resisting tumor invasionand metastasis, wherein the injection can be intravenous injection, intramuscular injection, intraperitoneal injection or subcutaneous injection. According to the tests, the Corilagin achieves good anti-tumor effect, more particularly, the Corilagin achieves good curative effect in both in-vitro tumor cell tests and in-vivo tumor animal models. Therefore, the Corilagin as a novel compound achieving the anti-tumor effect is applicable in preparing anti-tumor drugs.
Owner:XIAMEN TASMAN BIO TECH
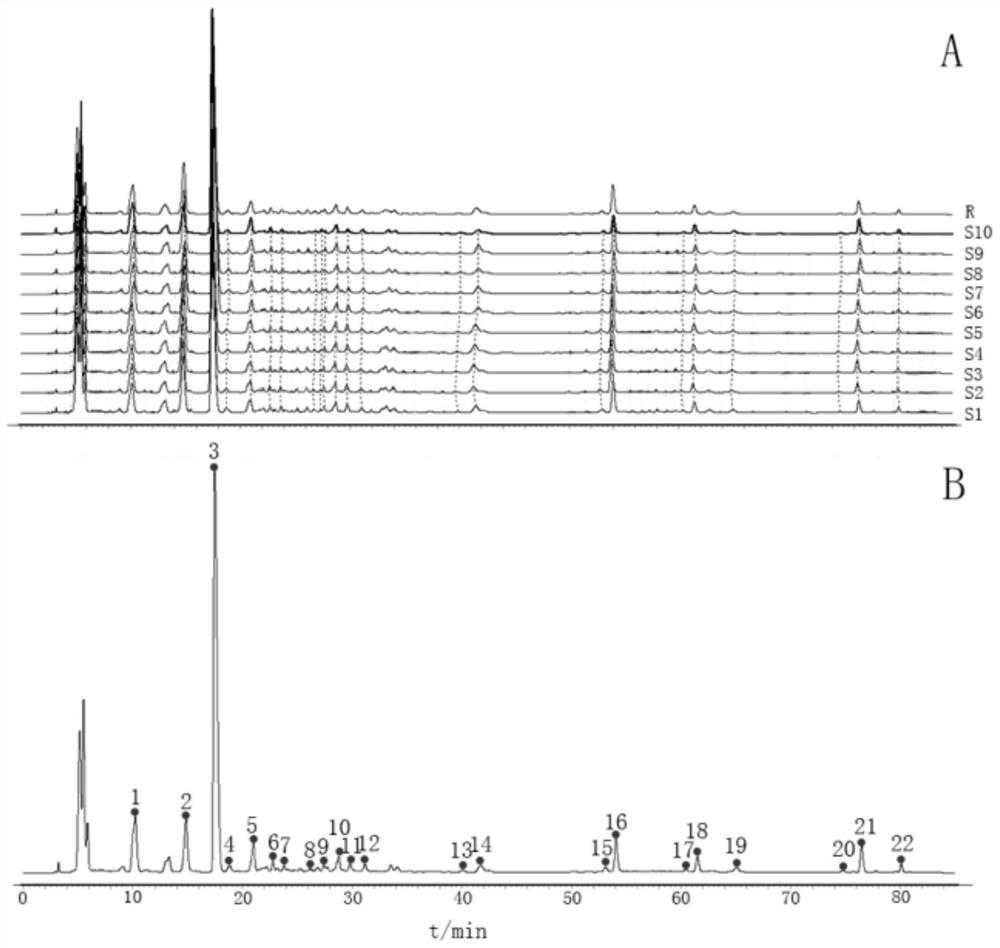
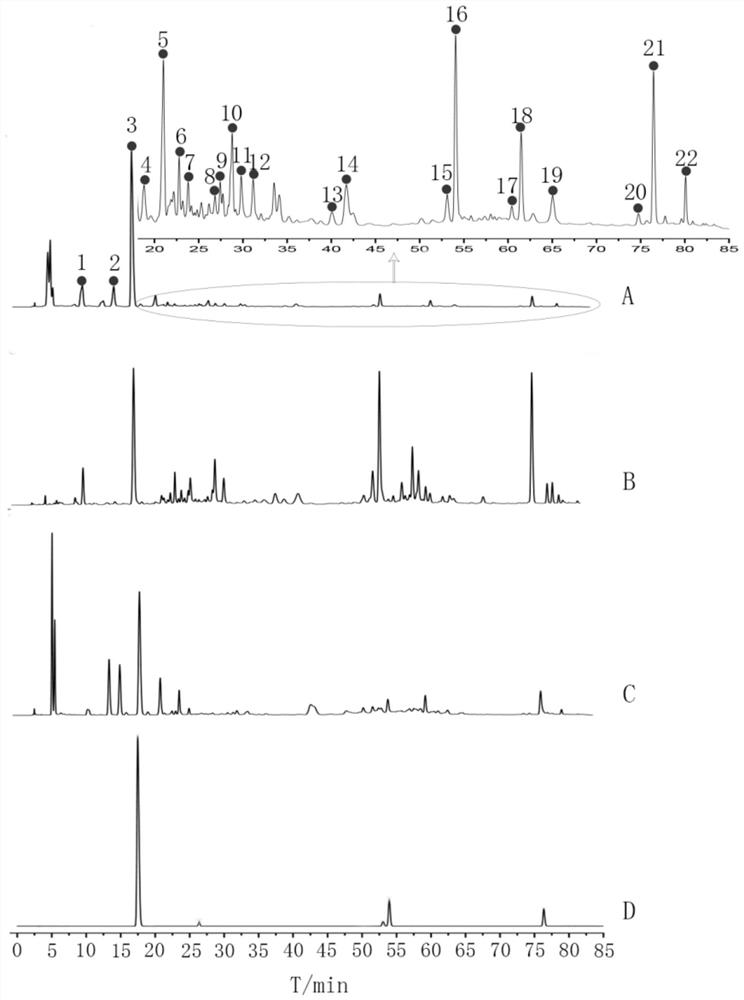
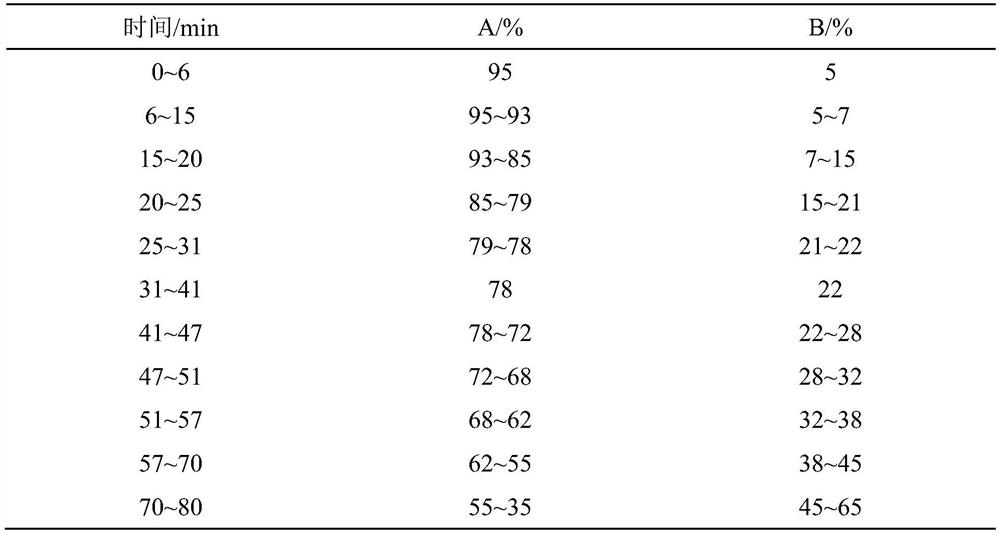
Construction method and application of medicine Terminalia fruit and Terminalia chebula Retz.var.tomentella Kurt. medicinal material specific chromatogram
ActiveCN111272904AFully respond to characteristic peak informationComprehensive evaluationComponent separationBiotechnologyMedicinal herbs
The invention relates to a construction method and application of a medicine Terminalia fruit and Terminalia chebula Retz.var.tomentella Kurt. medicinal material characteristic spectrum. The construction method of the medicine Terminalia fruit medicinal material specific chromatogram comprises the following steps: preparing a reference substance solution by taking gallic acid, ethyl gallate, corilagin, Chebulagic acid, myrobalan acid and chebulic acid as reference substances; dissolving a medicine Terminalia fruit in a solvent, performing ultrasonic extraction, filtering an extracting solution, and taking subsequent filtrate to obtain a medicine Terminalia fruit test solution; respectively carrying out ultra-high performance liquid chromatography detection on the reference substance solution and the medicine Terminalia fruit test solution. Chromatographic conditions of the ultra-high performance liquid chromatography comprise that a mobile phase A is acetonitrile, a mobile phase B is aphosphoric acid aqueous solution with a volume fraction of 0.1-0.3%, and an elution mode is gradient elution. The invention provides a comprehensive, effective and rapid method for controlling and evaluating the quality of the medicine terminalia fruit (collectively referred to as myrobalan).
Owner:GUANGDONG YIFANG PHARMA
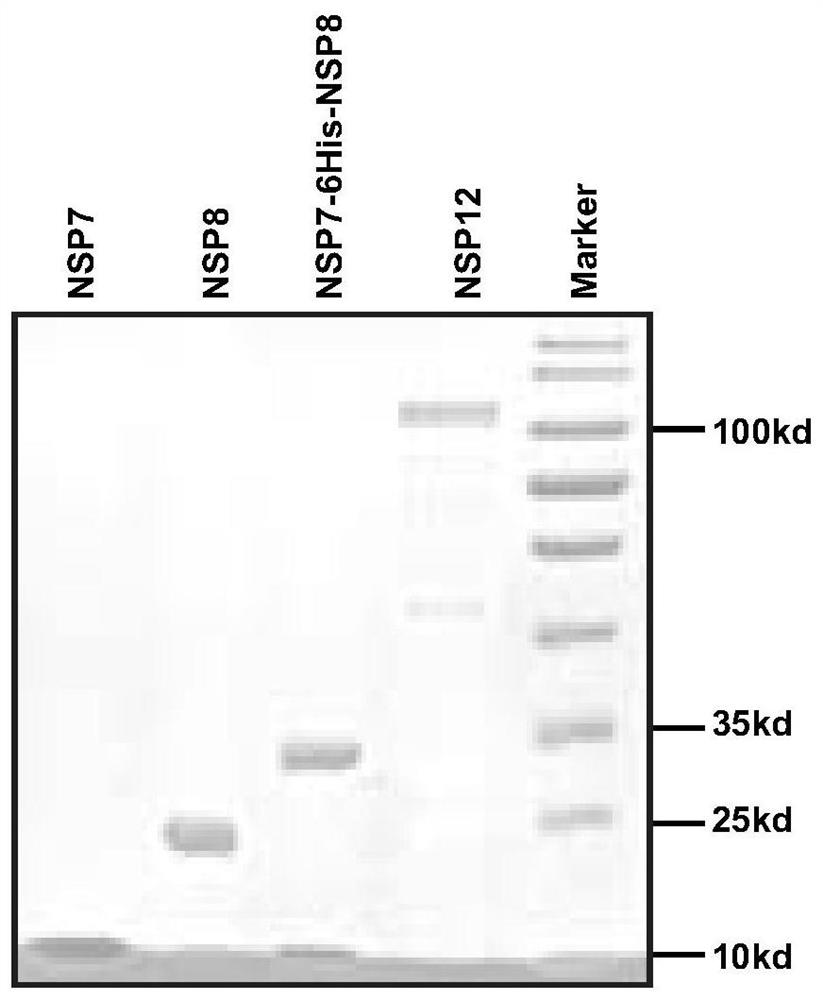
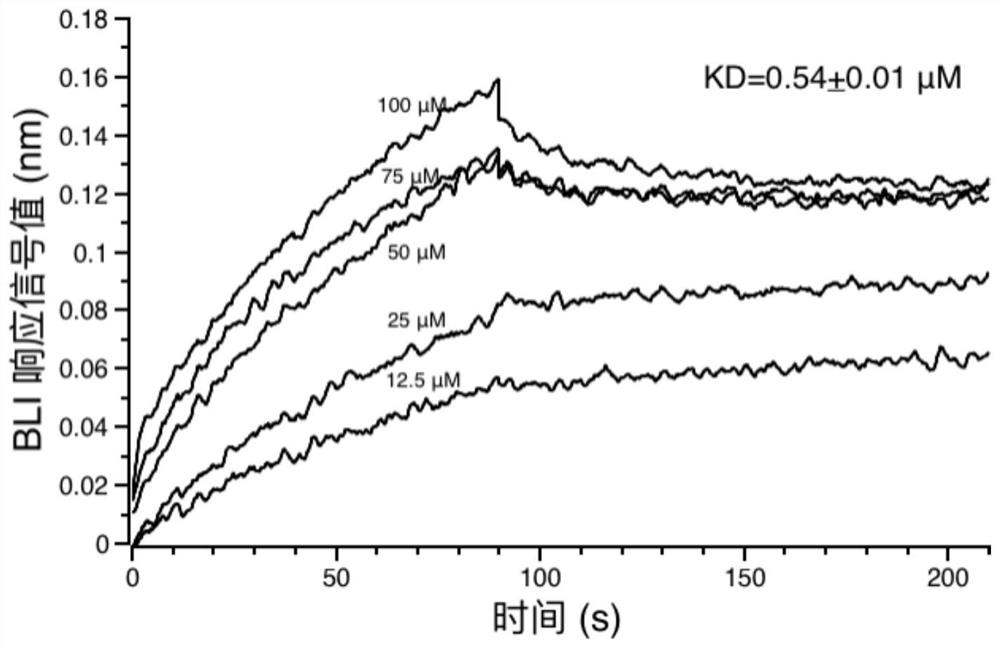
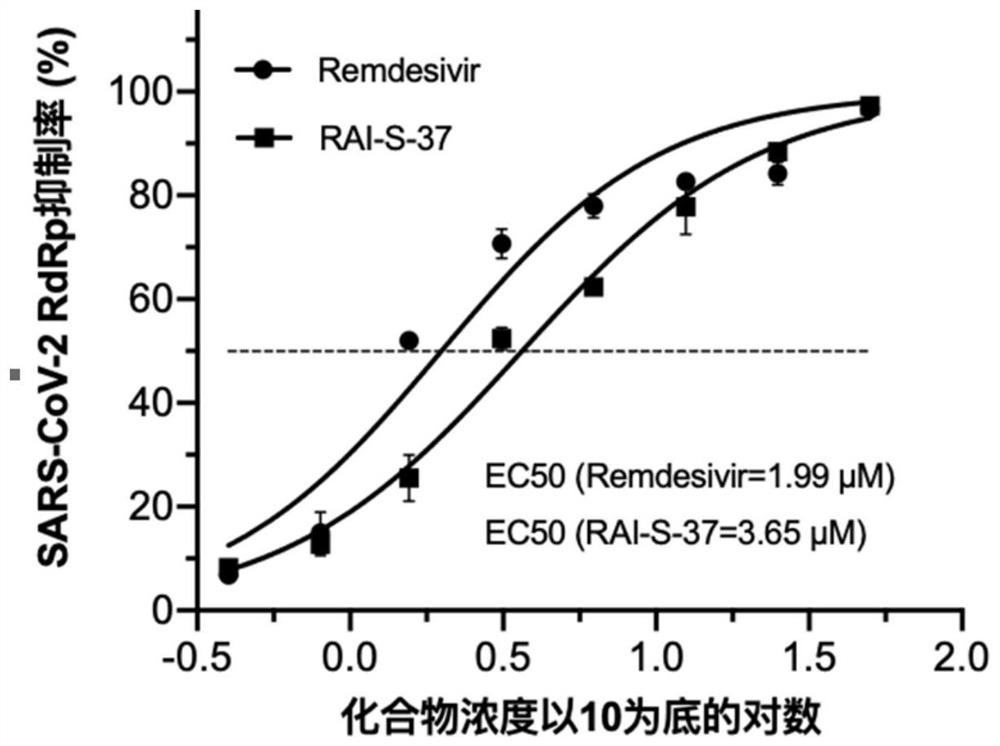
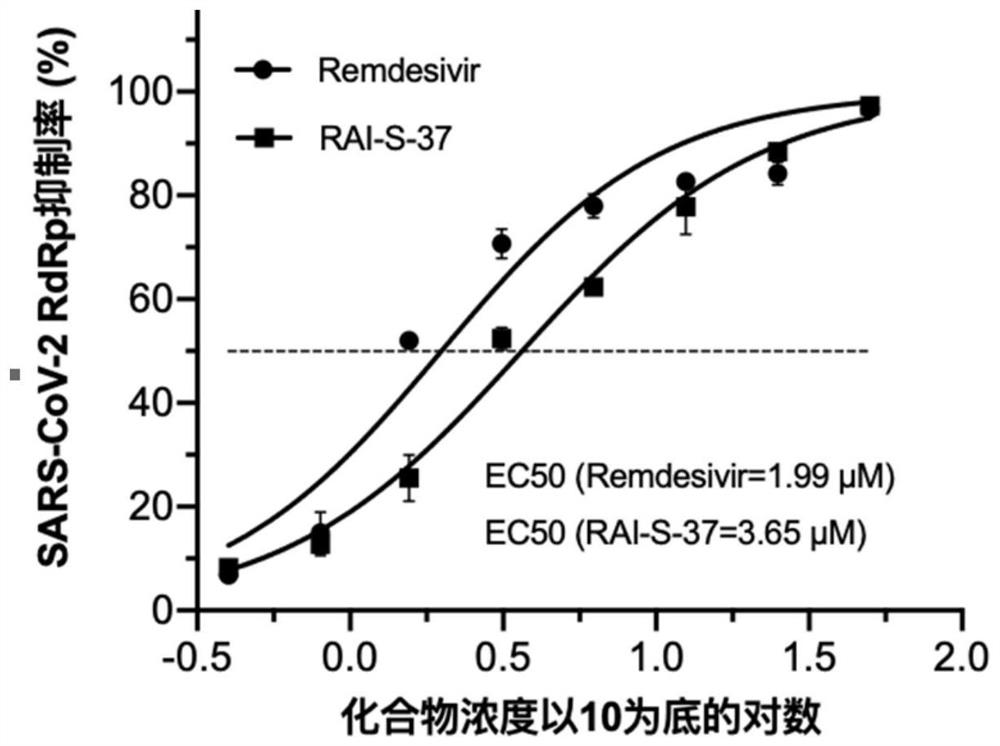
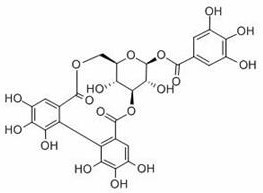
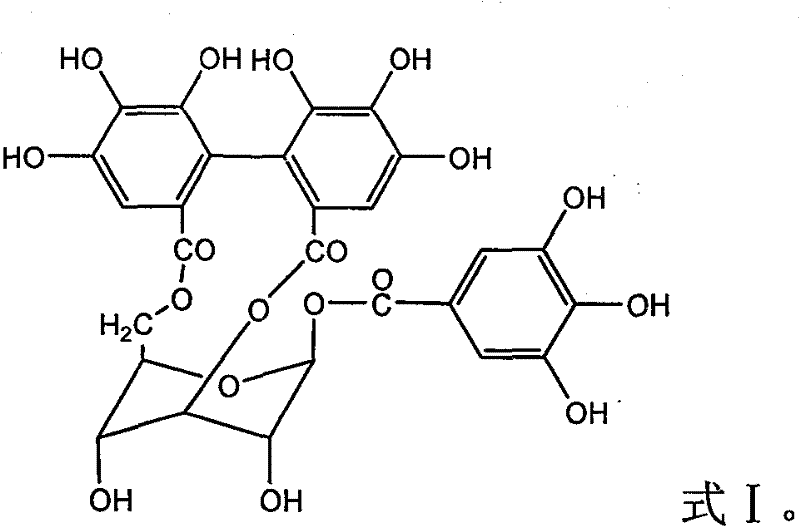
Application of corilagin in inhibition of coronavirus replication to play roles of anti-coronavirus drugs
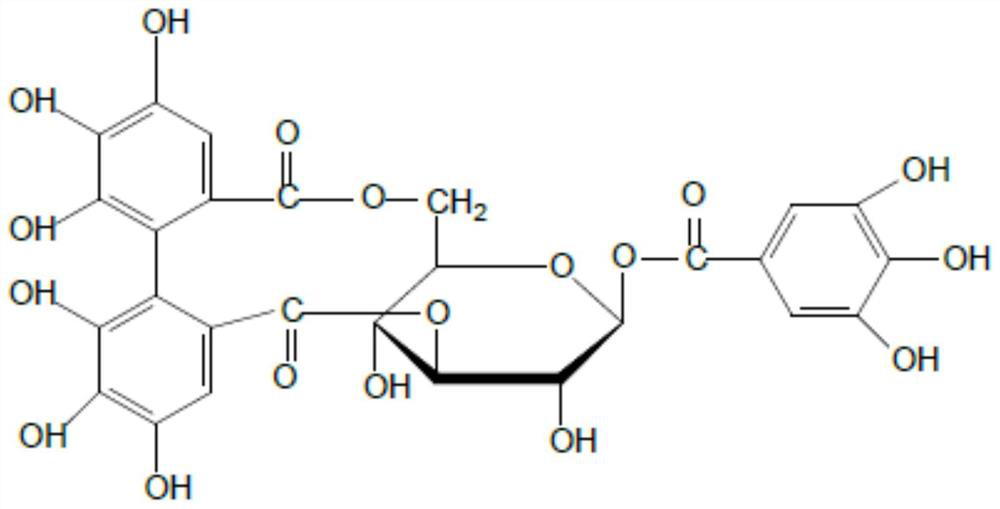
ActiveCN112451534AInhibition of replicationOrganic active ingredientsAntiviralsChemical compoundPharmaceutical drug
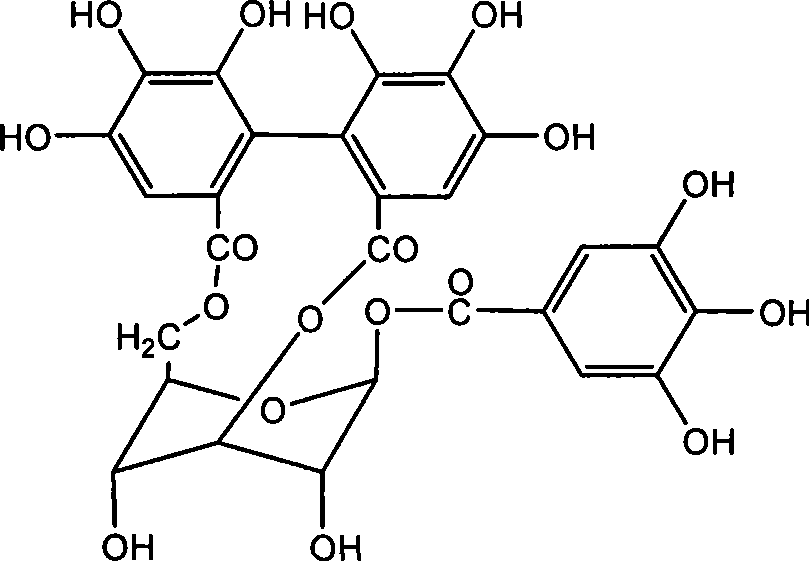
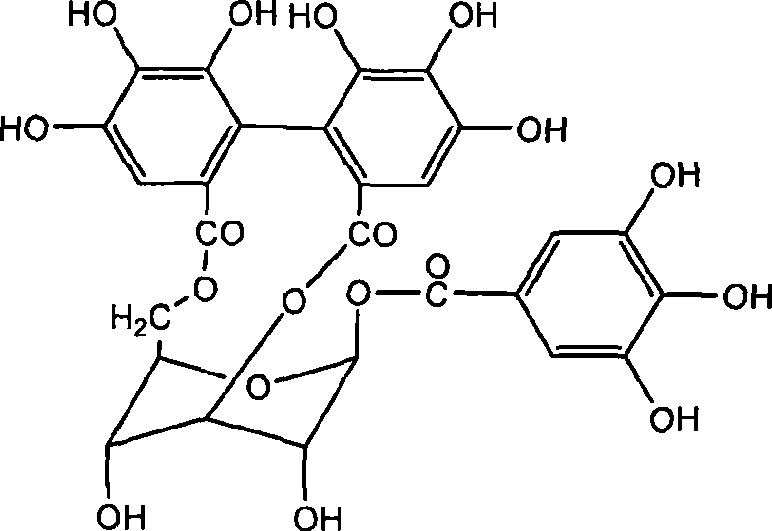
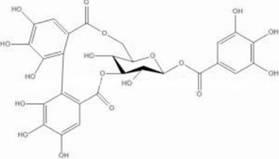
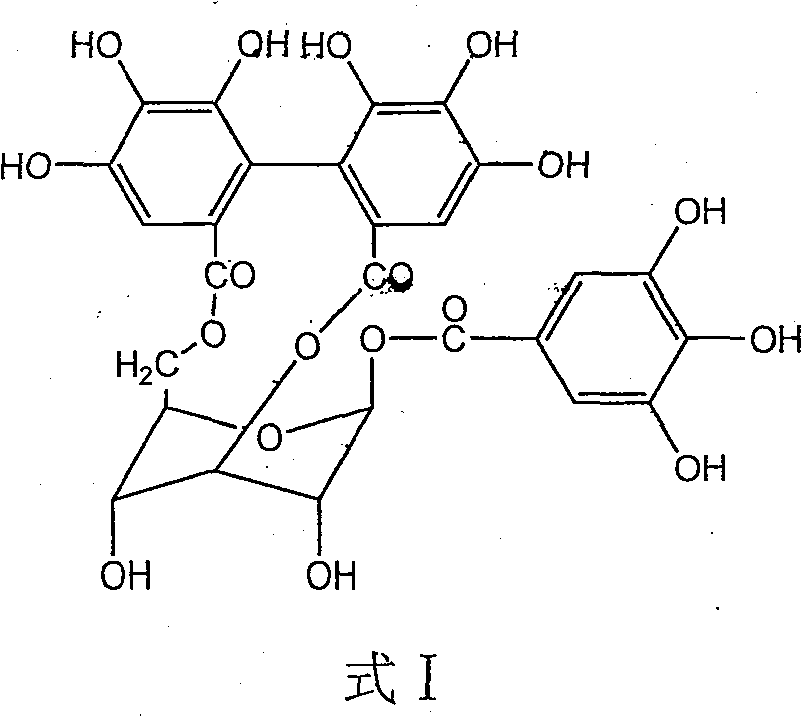
The invention discloses application of corilagin in inhibition of coronavirus replication to play roles of anti-coronavirus drugs. The structural formula of the corilagin (Chinese name, Kelilajing; English name, Corilagin; and CAS number, 23094-69-1) is shown in the formula (I). The invention provides application of a compound as shown in the formula (I) or pharmaceutically acceptable salt thereof. The application is as follows: (a) application in preparation of a coronavirus inhibitor; and (b) application in inhibition of coronavirus. According to the invention, experiments prove that the compound shown in the formula (I) can effectively inhibit replication of SARS-CoV-2 in vitro, has good anti-SARS-CoV-2 activity, and has an important application prospect for treating SARS-CoV-2 infection.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
New geranium extract
InactiveCN101613382AReduce secretionReduce ulcer indexEsterified saccharide compoundsOrganic active ingredientsDiseaseMedicine
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Preparation method of corilagin
InactiveCN102020683AHigh purityConducive to mass production operationsEsterified saccharide compoundsSugar derivativesWater basedPhyllanthus urinaria
The invention relates to a preparation method of corilagin, wherein the preparation method is simple and convenient in operation, less in pollution and low in energy consumption. The preparation method comprises the following process steps: taking coarse powder of phyllanthus urinaria; adding the coarse powder of phyllanthus urinaria into a CO2 supercritical extractor for extracting to obtain an extract; adding water for dissolving; filtering; adsorbing the filtered solution by macroporous absorption resin; eluting by ethanol; collecting the eluent; recovering the ethanol at the reduced pressure, and concentrating the ethanol; adding acetic acid into the concentrated solution for regulating the pH to 4-4.5; carrying out 6-10-grade counter-current extraction by a mixed solvent formed by mixing ethyl acetate and water based on the ratio of 10:2, wherein the volume ratio of the concentrated solution to the total dosage of the mixed solvent is (1:6)-10; mixing the extracted solutions; recovering the solvent at the reduced pressure; adding absolute ethanol for crystallizing; and separating the crystal, washing and drying to obtain a finished product. By using the method provided by the invention for preparing the corilagin, the product purity is high, and the industrialized amplification can be realized easily.
Owner:SUZHOU PAITENG BIOLOGICAL MEDICAL TECH
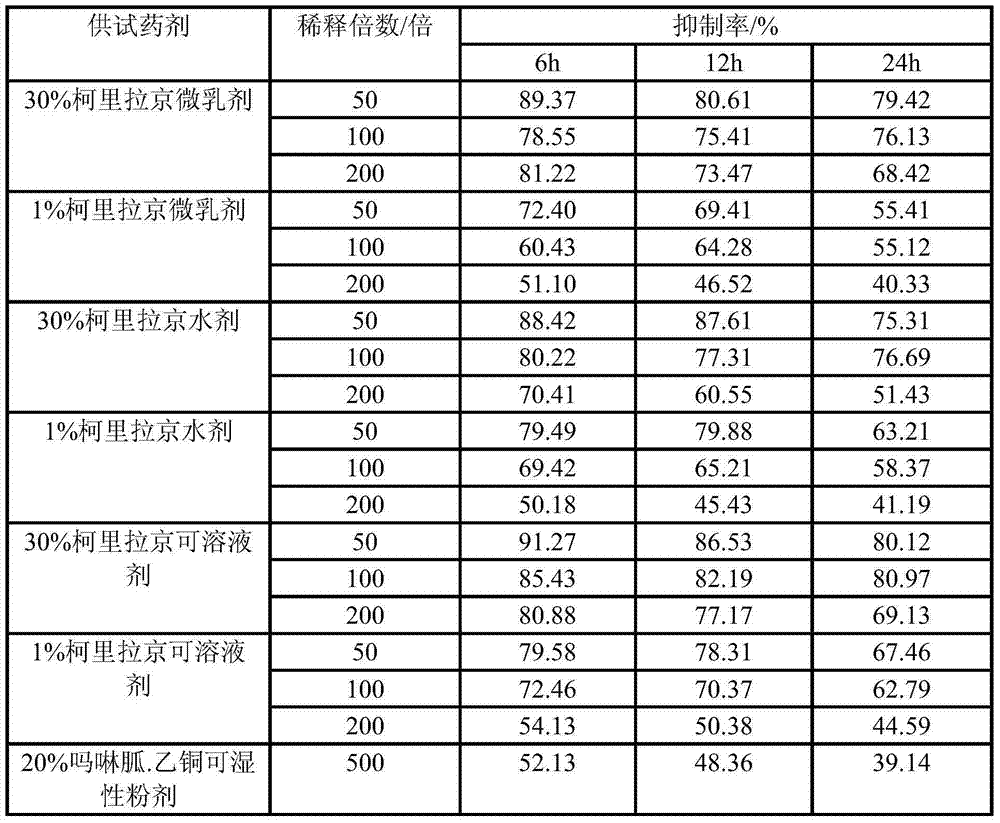
Plant virus resistant agent and preparation method thereof
ActiveCN103749490AHigh activityGood environmental compatibilityBiocideDisinfectantsDiseaseNicotiana tabacum
The invention discloses a plant virus resistant agent. The effective constituent of the prepared plant virus resistant agent is corilagin, and auxiliaries are assisted to prepare soluble concentrate, aqueous solution and microemulsion. The plant virus resistant agent is harmless to human, livestock, natural enemies of pests and other beneficial organisms, has good environment compatibility, has no residual hazard after use, has abundant resources, simple preparation method and low cost, and can be used for preventing plant virus diseases such as tobacco mosaic virus, cucumber mosaic virus and potato virus on tobacco, chili, tomato, cucurbita pepo, potato and other crops.
Owner:NORTHWEST A & F UNIV
Method for preparing corilagin through matsumura leafflower herb
InactiveCN102329344AReduce dosageFully extractedEsterified saccharide compoundsSugar derivativesChromatographic separationFiltration membrane
The invention discloses a method for preparing corilagin through a matsumura leafflower herb. The method comprises the steps of: crushing a matsumura leafflower herb medicinal material; performing Soxhlet extraction by adding 40-60 percent of ethanol; filtering an extracted solution; ultra-filtering by adding an ultra-filtration membrane system; concentrating a liquid permeating the ultra-filtration membrane system through a reverse osmosis membrane to obtain a concentrated liquid; regulating the pH value of the concentrated liquid to be pH 4-5; extracting through ethyl acetate; separating through a high-speed countercurrent chromatography after concentrating an extract liquid; collecting target components according to a chromatogram of an ultraviolet detector; and drying at a low temperature to obtain a product. By adopting the method for producing the corilagin, the energy consumption is low, the yield rate is high, and the product content is high.
Owner:苏州宝泽堂医药科技有限公司
The application of corilagin in inhibiting the replication of coronavirus to exert the function of anti-coronavirus drug
ActiveCN112451534BInhibition of replicationOrganic active ingredientsAntiviralsPharmaceutical drugPharmaceutical medicine
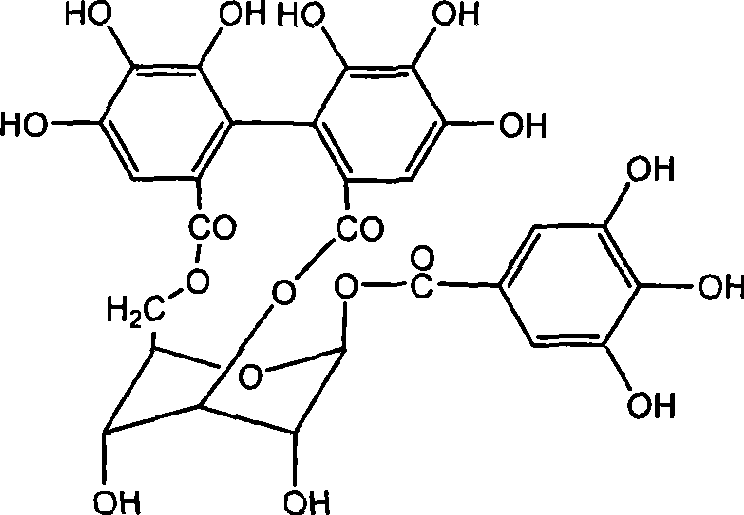
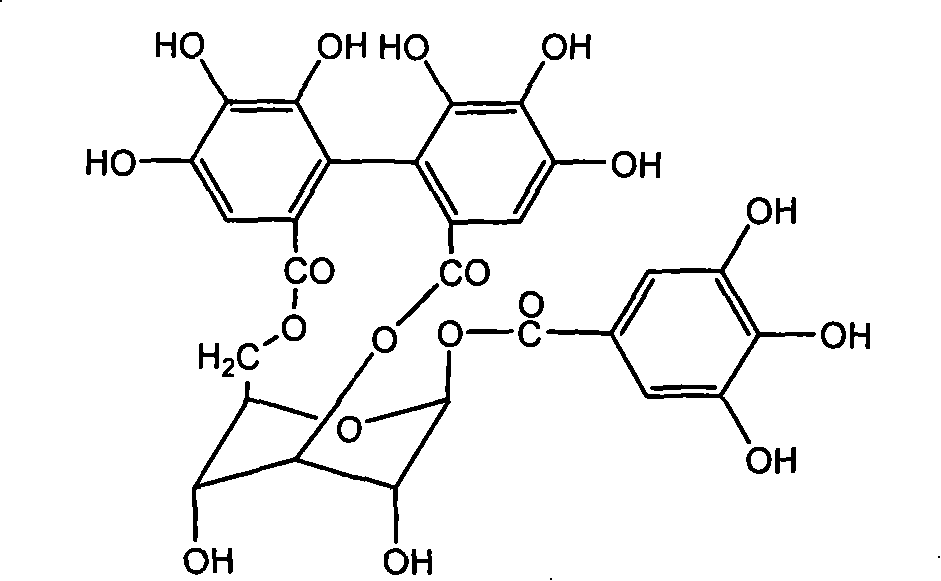
The invention discloses the application of corilagin in inhibiting the replication of coronavirus so as to exert the function of anti-coronavirus drug. Corilagin, the CAS number is 23094‑69‑1, the Chinese name is Corilagin, the English name is Corilagin, and the structural formula is shown in formula (I). The present invention provides the application of the compound represented by formula (I) or a pharmaceutically acceptable salt thereof, which is the following (a) or (b): (a) in the preparation of coronavirus inhibitors; (b) in Inhibition of coronavirus applications. The present invention proves through experiments that the compound represented by formula (I) can effectively inhibit the replication of SARS-CoV-2 in vitro, has good anti-SARS-CoV-2 activity, and has important application prospects for treating SARS-CoV-2 infection.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Plastic with function of seawater corrosion resistance
The invention relates to a plastic with a function of seawater corrosion resistance. The formula of the plastic comprises raw materials in parts by weight as follows: 80 parts of HDPE (high-density polyethylene), 11 parts of hydroxyethyl acrylate, 10 parts of vinyl acetate, 15 parts of Ni-Cr alloying powder, 2.5 parts of alginate polysaccharides, 2.5 parts of quaternary ammonium salt of chitosan, 2 parts of isobarbaloin, 3 parts of corilagin and 5 parts of plant fiber. The plastic has the function of seawater corrosion resistance and can be used for a building at the sea.
Owner:QINGDAO KECHUANG PLASTIC MACHINERY
Application of Corilagin in preparation of medicine for treating burns and scalds
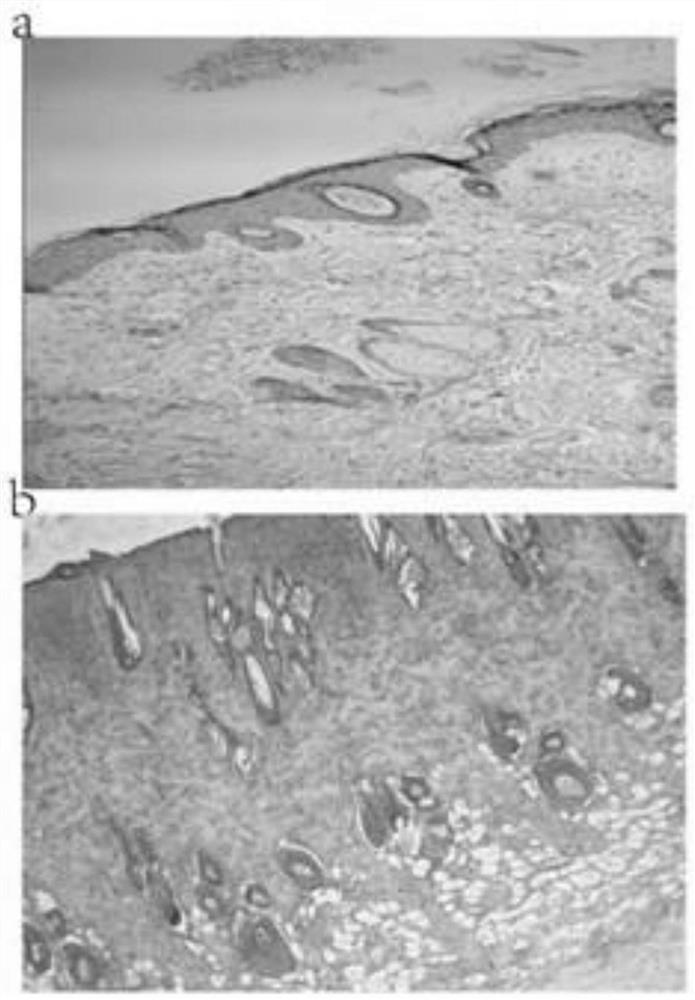
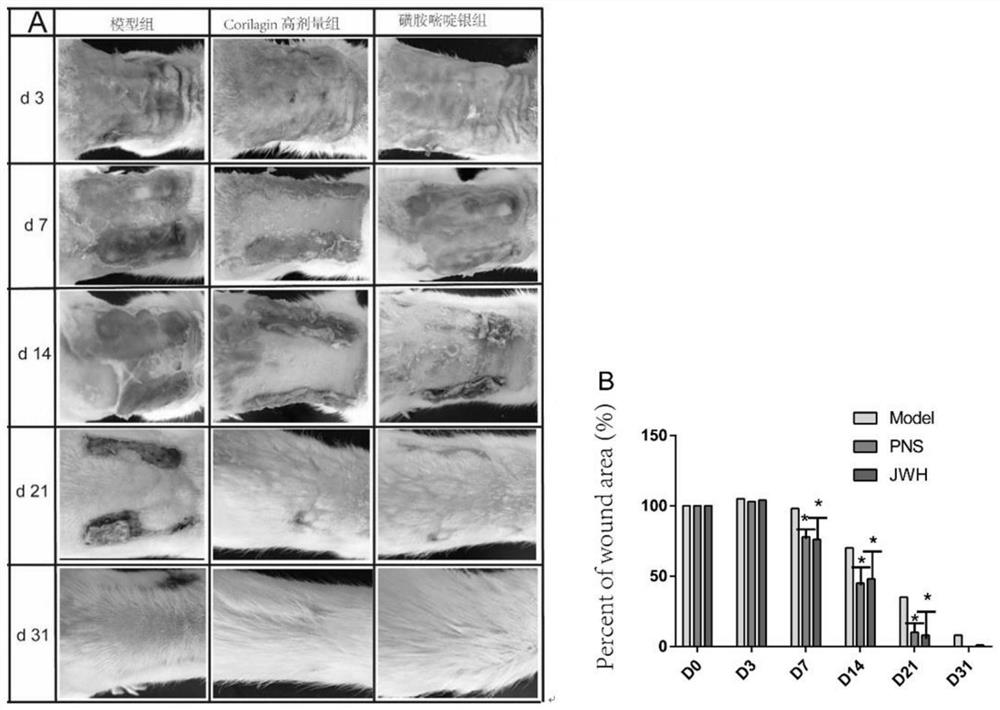
InactiveCN113368119AShorten the timeImprove healing rateOrganic active ingredientsDermatological disorderTreatment effectSulfanilamide
The invention discloses application of Corilagin in preparation of a medicine for treating burns and scalds. The Corilagin is one of a Corilagin monomer or a Corilagin salt or a combination of the Corilagin monomer and the Corilagin salt. The Corilagin is externally used and reduces the growth of bacteria on the wound surfaces of burns and scalds by inhibiting the generation and release of inflammatory mediators to relieve the inflammatory response of the wound surfaces. Corilagin (8g / kg) reduces the inflammatory reaction of the wound surfaces by inhibiting the generation and release of inflammatory mediators, so as to reduce the growth of bacteria on the wound surfaces of the burns (scalds); and meanwhile, the protein and gene expression levels of PDGF-BB and PDGFR-beta are up-regulated, so that the expression peak is advanced, the decrustation and hair growing time of rats is shortened, and the healing rate of wound surfaces is improved. The Corilagin has an obvious treatment effect on the burns (scalds), and the curative effect of the Corilagin is even superior to that of a clinical first-line medicine 1% sulfadiazine silver.
Owner:KUNMING MEDICAL UNIVERSITY
Method for extracting corilagin from emblic leafflower fruit and application of corilagin
PendingCN114591381AFully recycleInhibitory activityEsterified saccharide compoundsBiocideBiotechnologySephadex
The invention discloses a method for extracting corilagin from phyllanthus emblica and application of the corilagin, and the method comprises the following steps: extracting phyllanthus emblica pomace with ethanol to obtain an ethanol layer crude extract; dissolving the ethanol layer crude extract with methanol water, separating and purifying with a reversed-phase C18 column, and collecting the 31-36th min component as a component EA-2; dissolving the component EA-2 by using methanol, separating and purifying by using Sephadex LH-20, and collecting the component from 3180 to 4620 minutes to obtain the component EA-2-2; and dissolving the component EA-2-2 with methanol, purifying the dissolved component EA-2-2 with a high performance liquid chromatograph, and collecting the 14-16 min component as the component EA-2-2-1. Therefore, the phyllanthus emblica pomace can be fully recycled, waste is avoided, the obtained component is EA-2-2-1 corilagin, and the compound can efficiently antagonize potato streptomyces scabies and has important application value in prevention and treatment of potato scab.
Owner:JIMEI UNIV
Novel use of corilagin
InactiveCN101461816BReduce secretionReduce acidityDigestive systemCarbohydrate active ingredientsDiseasePeptic ulcer
The invention relates to novel application of corilagin or a pharmaceutically acceptable corilagin salt, in particular to application of the corilagin or the pharmaceutically acceptable corilagin salt in treating or preventing diseases related to gastric acid hypersecretion such as hyperchlorhydria, peptic ulcer and chronic gastritis.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
The Extraction and Separation Method of Corilagin and Its Application
InactiveCN104447896BEasy to recycleReduce oil soluble componentsEsterified saccharide compoundsCosmetic preparationsAqueous acetonePolyamide
The invention discloses an extraction-separation method and an application of corilagin. The method comprises the steps of treating longan kernel powder once by using anhydrous acetone and extracting by using acetone aqueous solution again for the second time; uniformly mixing a proper amount of chromatography polyamide with the acetone aqueous solution, recycling acetone and applying to a polyamide chromatography column; eluting by sequentially using pure water, methyl alcohol aqueous solutions with different concentrations and methyl alcohol; gathering 40-60 percent of methyl alcohol eluant in a separate bottle form, condensing and separating out corilagin crystals; mixing mother liquid with the rest of eluant containing corilagin, condensing to be dry, dissolving into methyl alcohol and applying to a polydextran gel LH-20 column; eluting by methyl alcohol; gathering eluant in a separate bottle form and standing; mixing with the eluants in which corilagin crystals are separated out; standing again until the orilagin crystals which are separated out are not increased; mixing the obtained corilagin crystals with corilagin crystals obtained by chromatography polyamide; performing recrystallization by using ethyl alcohol to obtain corilagin with high purity. The corilagin subjected to extraction and separation has a function of inhibiting tyrosinase.
Owner:GUANGDONG FOOD & DRUG VOCATIONAL COLLEGE
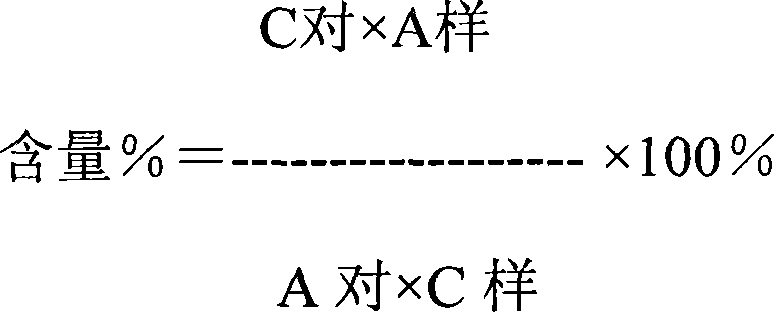
Quality control method and application of P. urinaria L. capsule
PendingCN113447598ARaise quality standardsHarvest time controllableComponent separationDigestive systemBiotechnologyModern medicine
The invention relates to a quality control method and application of P. urinaria L. capsules, and belongs to the technical field of biological medicines. Control is carried out in three aspects of raw material control, preparation method quality control and product quality control. According to the invention, modern medicine production management is integrated into quality control, the quality of medicines rely on production, and a perfect quality standard and a relatively high and reasonable method and standard for detecting the content of the active ingredient corilagin are established through whole-process quality control from the source. Pharmacological tests prove that the P. urinaria L. capsules have the effects of preventing and treating hepatic cell injury and hepatic fibrosis, and have wide application prospects.
Owner:朱明浩
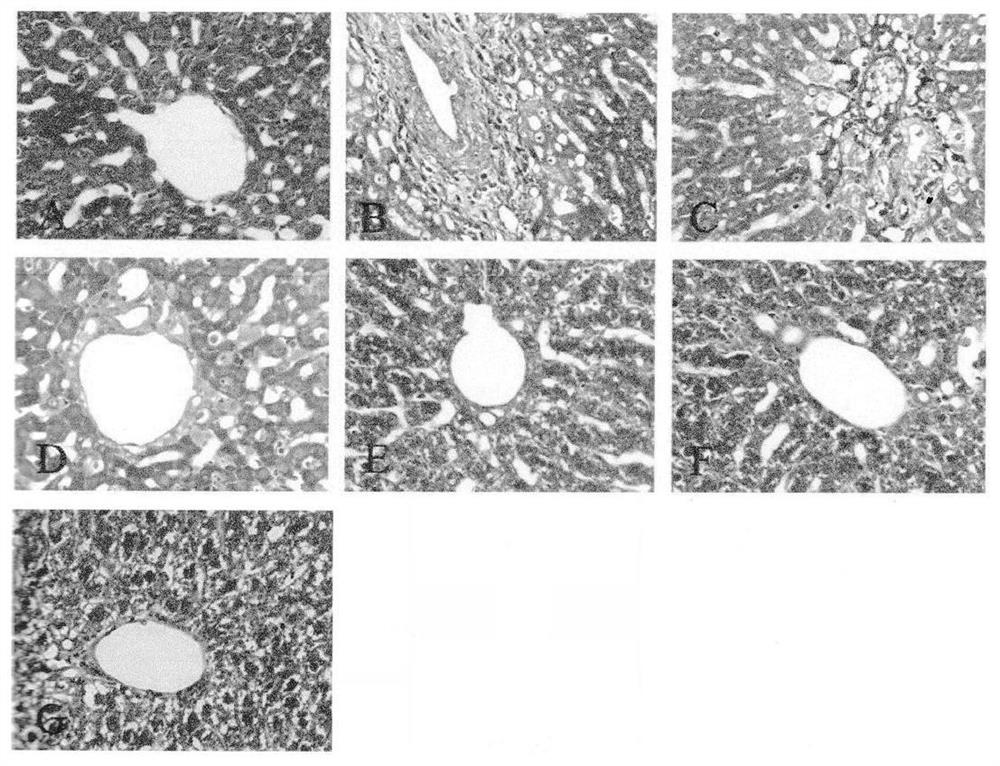
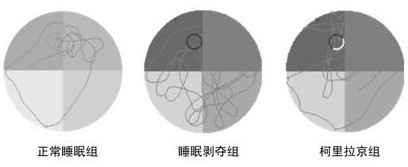
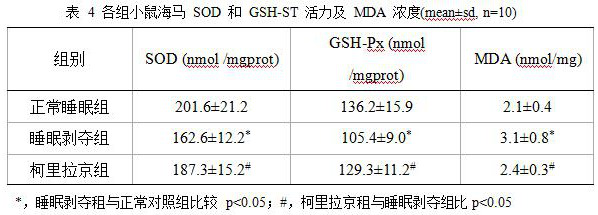
New application of corylagin in the preparation of anti-sleep deprivation injury drugs
ActiveCN110613721BImprove physical conditionAvoid damageOrganic active ingredientsNervous disorderOxidative stressPharmaceutical drug
The present invention relates to the new application of Corilagin in the preparation of sleep deprivation medicine. The mouse sleep model was established by using the small platform water environment method, and the Morris maze / platform experiment was carried out to investigate the learning and memory ability, and further detected the oxidoreductase and cholinergic indicators of the hippocampal tissue. The results showed that the administration of corilagin significantly improved the behavioral disorders of sleep-deprived mice, relieved the oxidative stress damage of hippocampus tissue, and strengthened the cholinergic system. It is suggested that Corilagin can significantly improve the behavior of sleep-deprived mice, and has a certain application prospect in coping with high-intensity continuous operations.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Traditional Chinese medicine component prescription for improving pig immunosuppressive state and its preparation method and application
ActiveCN108685933BInhibition releaseEnhance phagocytosisOrganic active ingredientsPharmaceutical delivery mechanismBiotechnologyDisease
Owner:JIANGSU ACAD OF AGRI SCI
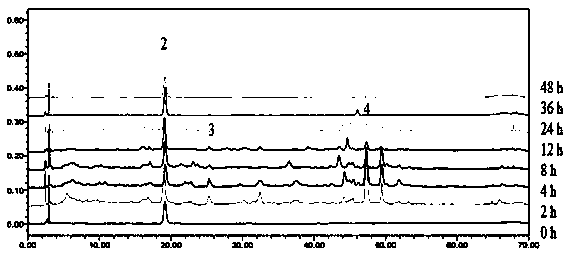
Method for determining contents of index components in bile after oral administration of emblic leafflower fruit tannin part and separating and identifying 30 chemical components
The invention provides a method for detecting chemical substances in bile after oral administration of an emblic leafflower fruit tannin part. The method comprises the following steps: collecting a bile sample; pretreating the bile sample; determining the contents of index components including gallic acid, corilagin and ellagic acid in bile; and simultaneously separating and identifying 6 prototype components and 24 metabolites in bile. The method has the advantages of being good in separation degree, simple, convenient, accurate, high in sensitivity, good in reproducibility, large in separation and identification number and the like, and can effectively evaluate the metabolism and excretion rules of the oral emblic leafflower fruit tannin part in bile.
Owner:BEIJING UNIV OF CHINESE MEDICINE
A quality detection method for Sanle Porridge Oral Liquid with both qualitative and quantitative evaluation
ActiveCN109521103BEasy to separateMonitor qualityComponent separationHplc fingerprintGallic acid ester
The invention discloses an HPLC fingerprint spectrum detection method of the Sanle pulp oral liquid. The fingerprint of the invention has many peaks, good separation, good repeatability and high precision, which provides an effective guarantee for comprehensively monitoring the quality of the Sanle pulp oral liquid. Utilizing the extraction method and chromatographic conditions of the fingerprint detection method of the present invention, the contents of five active ingredients gallic acid, gallocatechin, epicatechin, corilagin and ellagic acid in the Sanle pulp oral liquid can also be measured , Realized the dual research on the qualitative and quantitative aspects of Sanle Porridge Oral Liquid.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
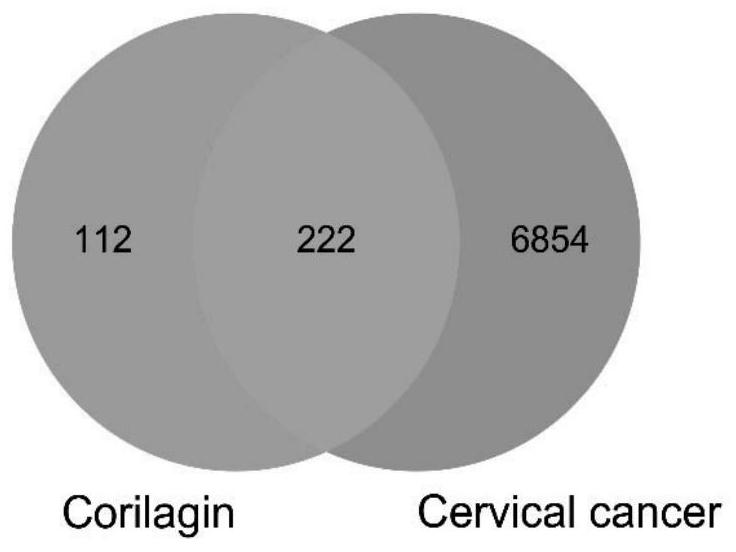
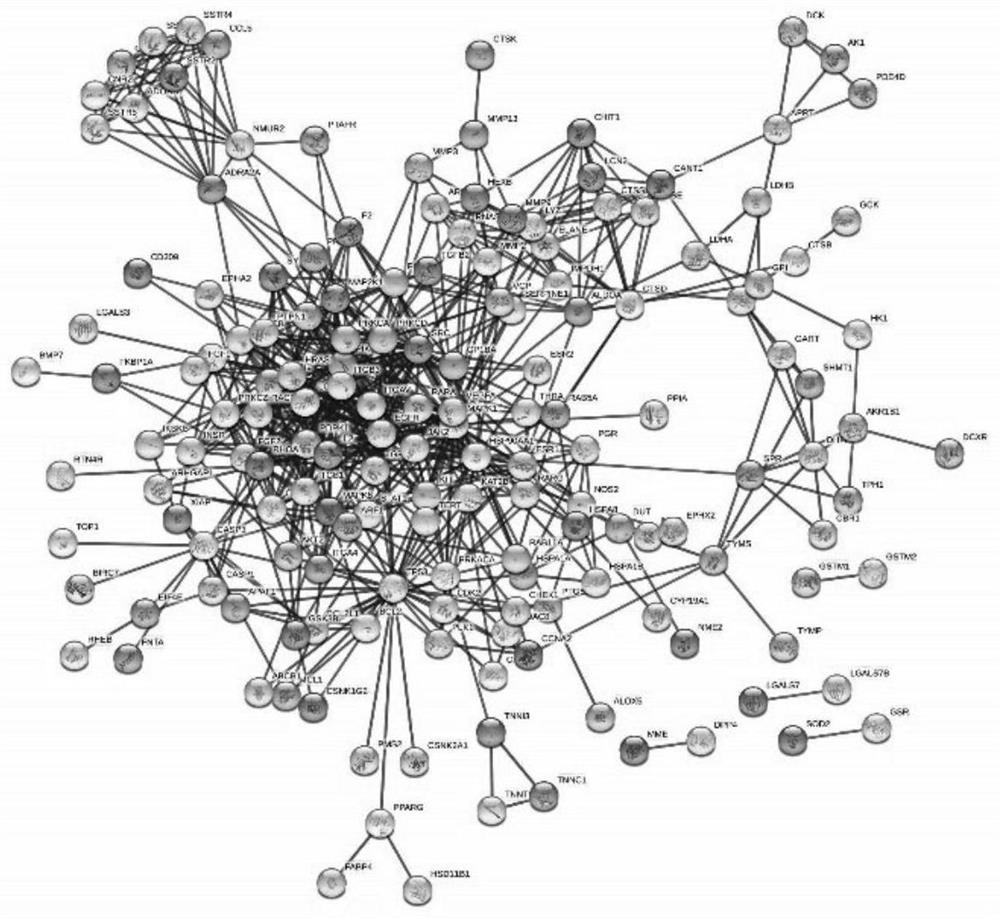
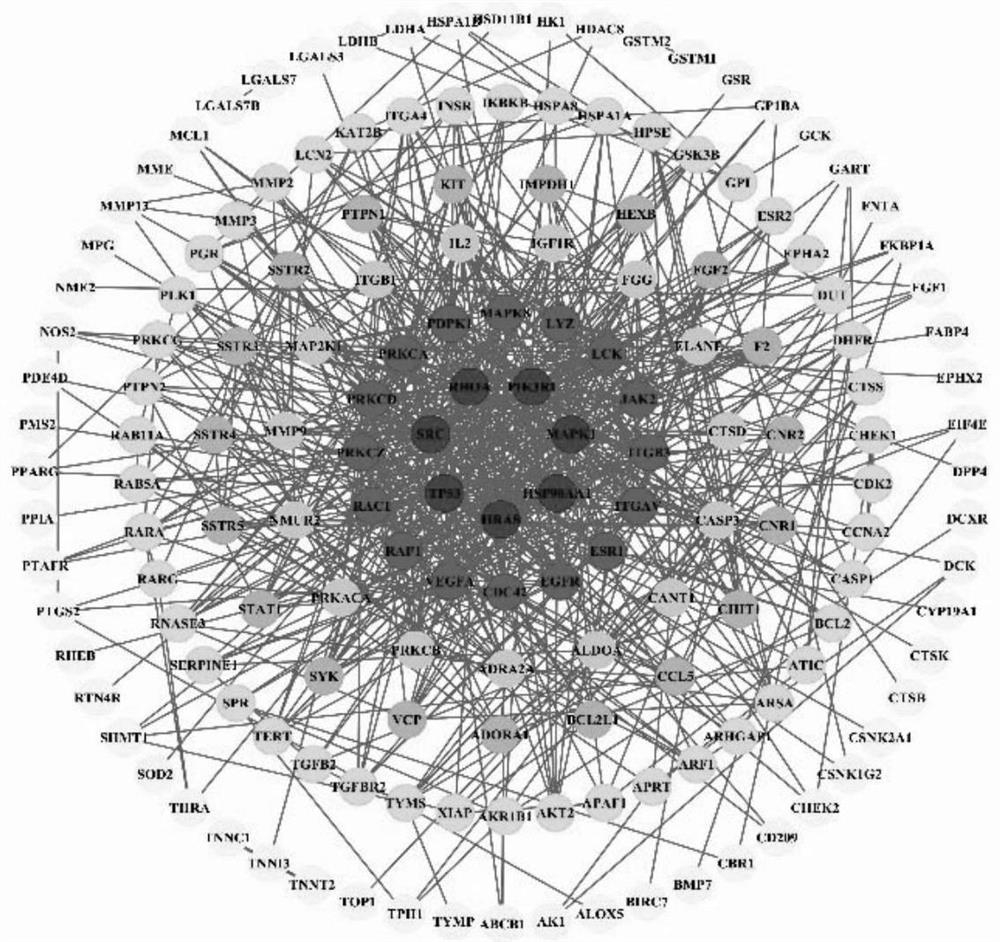
Method for analyzing anti-cervical cancer action mechanism of corilagin based on network pharmacology and molecular docking
PendingCN113936811AReduce R&D costsImprove screening efficiencyMedical data miningMolecular designDiseasePharmacometrics
The invention belongs to the field of biomedicine, and relates to a method for analyzing an anti-cervical cancer action mechanism of corilagin based on network pharmacology and molecular docking. The method comprises the following steps: S1, screening active component action targets; s2, identifying disease candidate targets; s3, constructing a component target-disease target network and screening core targets; s4, performing enrichment analysis on the core target spot to discuss the action mechanism of the medicinal components; s5, constructing and visualizing a 'component-target-pathway-disease' network; and S6, carrying out molecular docking on the corilagin anti-cervical cancer key target. A network pharmacology technology is utilized to construct the corilagin anti-cervical cancer'drug ingredient-target-disease' network diagram for the first time, and how the active ingredient corilagin performs the drug effect through 'multi-target, multi-path and multi-path' combined regulation and control is revealed.
Owner:WUHAN POLYTECHNIC UNIVERSITY
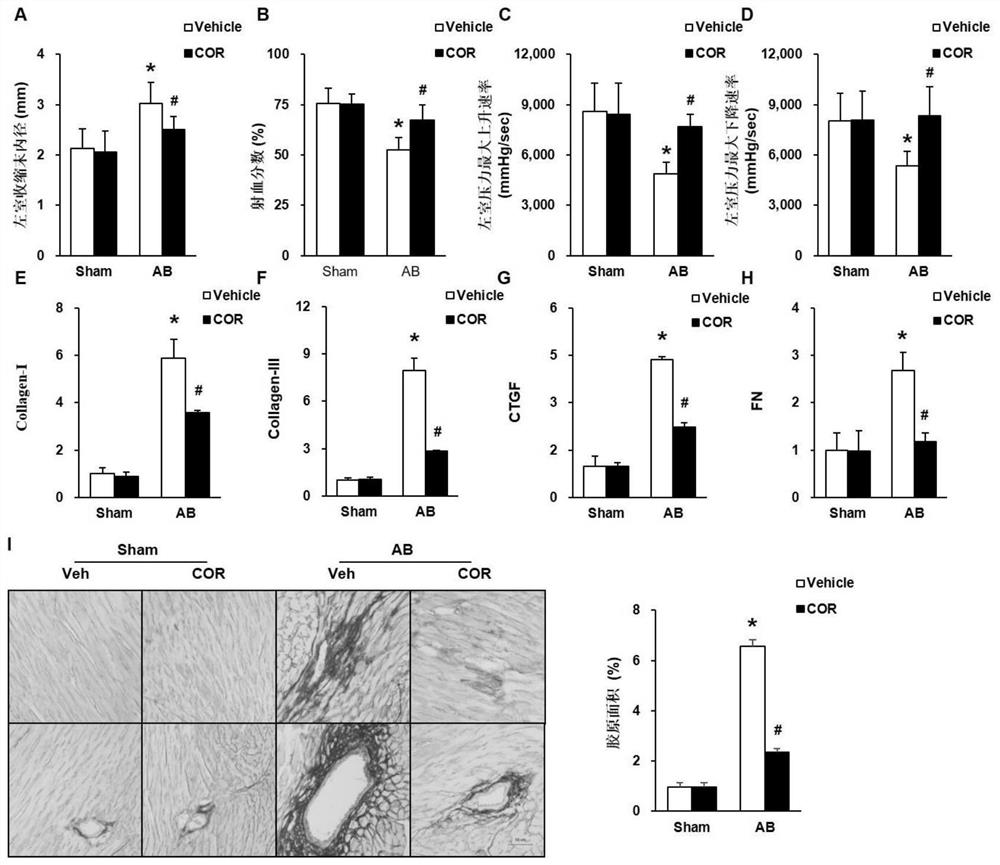
Use of Corilagin in the preparation of anti-myocardial fibrosis drugs
ActiveCN109674804BInhibit myocardial fibrosisAlleviate deterioration of heart functionOrganic active ingredientsCardiovascular disorderSide effectInhibitory receptors
The invention discloses the use of corilagin in the preparation of anti-myocardial fibrosis medicine. It belongs to the technical field of biomedicine. The present invention conducts experimental research by constructing an in vivo myocardial fibrosis model caused by mouse aortic ligation and an in vitro myocardial fibrosis model of rat suckling rat CFs induced by TGF-β, and the results show that corilagin can regulate the polarization of macrophages Thereby inhibiting inflammation, while down-regulating IL-4 receptors to avoid excessive production of TGF-β, and reducing the activation of myocardial fibroblasts by inhibiting the expression of TGF-β receptors, thereby reducing collagen content and reducing myocardial fibrosis. The present invention proposes that corilagin can reduce myocardial fibrosis caused by long-term pressure load, protect heart tissue, inhibit or delay the deterioration of cardiac function, has a significant effect in anti-myocardial fibrosis, and has few side effects, and can be used to prepare anti-myocardial fibrosis drugs .
Owner:WUHAN UNIV
New geranium extract
InactiveCN101613382BSimple preparation processReduce secretionEsterified saccharide compoundsOrganic active ingredientsDiseaseTraditional medicine
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Construction method and application of characteristic maps of Myrobalan and Myrobalan tomentosa
ActiveCN111272904BFully respond to characteristic peak informationComprehensive evaluationComponent separationMedicinal herbsGallic acid ester
The invention relates to a construction method and application of a characteristic map of Myrobalan and Myrobalan officinalis medicinal materials. The construction method of the characteristic map of the chebula medicinal material comprises the following steps: using gallic acid, ethyl gallate, corilagin, chebula acid, chebulic acid and chebulinic acid as reference substances, preparing a reference substance solution; taking chebula Medicinal material, solubilized and dissolved, ultrasonically extracted, the extract was filtered, and the filtrate was taken to obtain the test solution of Myrobalan; the reference substance solution and the test solution of Myrobalan were respectively carried out by ultra-high performance liquid chromatography; the ultra-high performance liquid The chromatographic conditions of the phase chromatography include: mobile phase A is acetonitrile, mobile phase B is phosphoric acid aqueous solution with a volume fraction of 0.1% to 0.3%, and the elution method is gradient elution. The invention provides a relatively comprehensive, effective and fast method for the quality control and evaluation of the medicinal materials of Myrobalan (collectively referred to as).
Owner:GUANGDONG YIFANG PHARMA
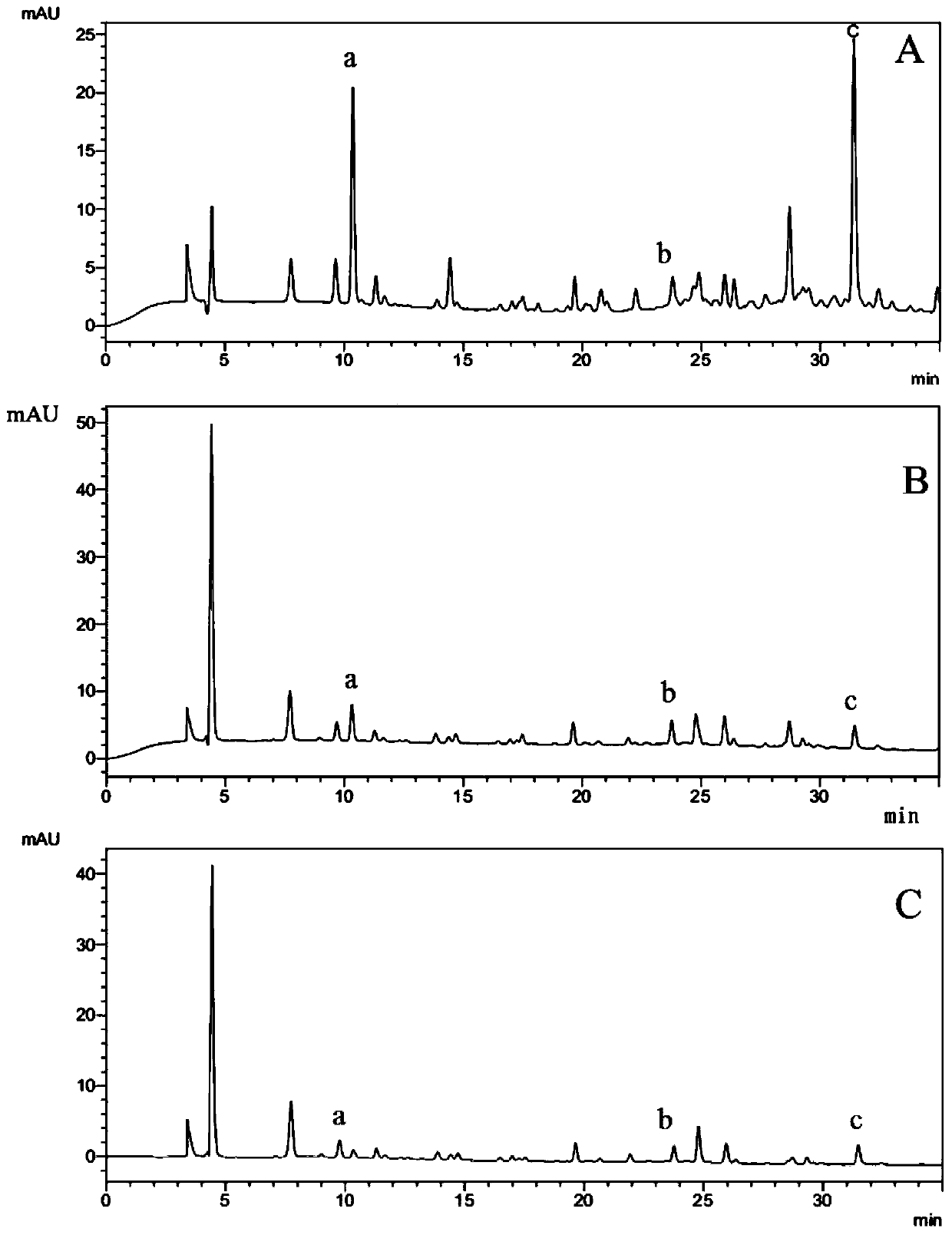
Phyllanthus niruri medicinal material fingerprint establishing method and standard fingerprint thereof
InactiveCN102331458ASignificant advantagesSignificant useComponent separationHplc fingerprintTest sample
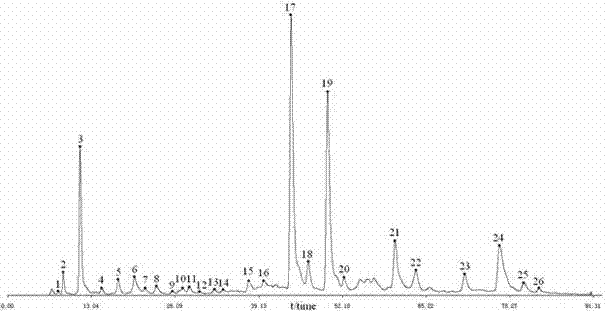
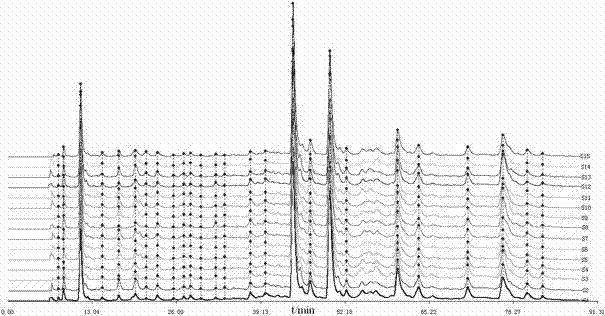
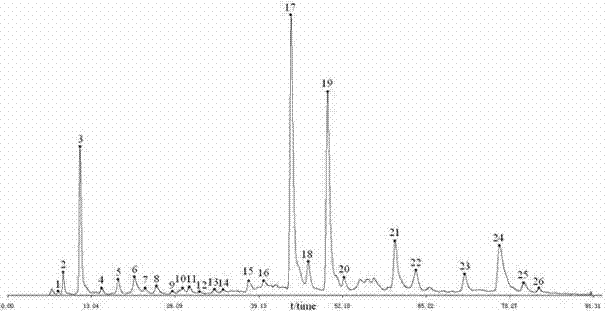
The invention discloses a phyllanthus niruri medicinal material efficient liquid phase fingerprint establishing method and a standard fingerprint thereof. Gallic acid and Corilagin are used as comparison products, HPLC (High Performance Liquid Chromatography) detection is carried out to a comparison product solution and a test sample solution under the same chromatograph condition, gradient elution is carried out, and a chromatogram is recorded to obtain the standard fingerprint. Through the comparison of the HPLC fingerprints of 15 batches of phyllanthus niruri medicinal materials, 26 common characteristic peaks are determined, and the common characteristic peaks constitute the fingerprint characteristics of the phyllanthus niruri medicinal materials and can be used as the standard fingerprints of the phyllanthus niruri medicinal materials. The invention has the characteristics of simple method, good repeatability, a large number of characteristic peaks, good accuracy and reliability and the like, and the established standard fingerprints can effectively represent the quality of the phyllanthus niruri medicinal materials and provide theoretical and practical foundations for perfecting the quality evaluation system of the phyllanthus niruri medicinal materials.
Owner:中国医学科学院药用植物研究所云南分所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com