Quality control method and application of P. urinaria L. capsule
A quality control method, capsule technology, applied in the field of biomedicine, to achieve the effect of wide application prospects, correct supply and demand, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: raw material control
[0084] The medicinal material of Phyllostachys urinaria used conforms to the 2005 edition of Yunnan Provincial Standards for Chinese Medicinal Materials: Yun YCBZ0010-2005, and was identified as P.urinaria L. by Kunming Institute of Botany, Chinese Academy of Sciences.
[0085] This artificially cultivated variety is an excellent variety that has been domesticated and selected from wild products distributed in Yunnan. Among them, the content of Corilagin is ≥0.8‰; it is better than wild varieties. Its yield can reach 2200kg / mu (medicinal material ~ 440kg / mu).
[0086] According to the requirements of GAP, there is no pollution source in the surrounding area (within 1km), the soil is loose and breathable, relatively fertile, sandy soil is better, pH5.8-7; sunny; farmyard manure is mainly used on the plot, and appropriate amount of superphosphate and urea, Deep plow and mix well to make a bed; the whole plot is high, with a width of ...
Embodiment 2
[0092] Embodiment 2: preparation method quality control
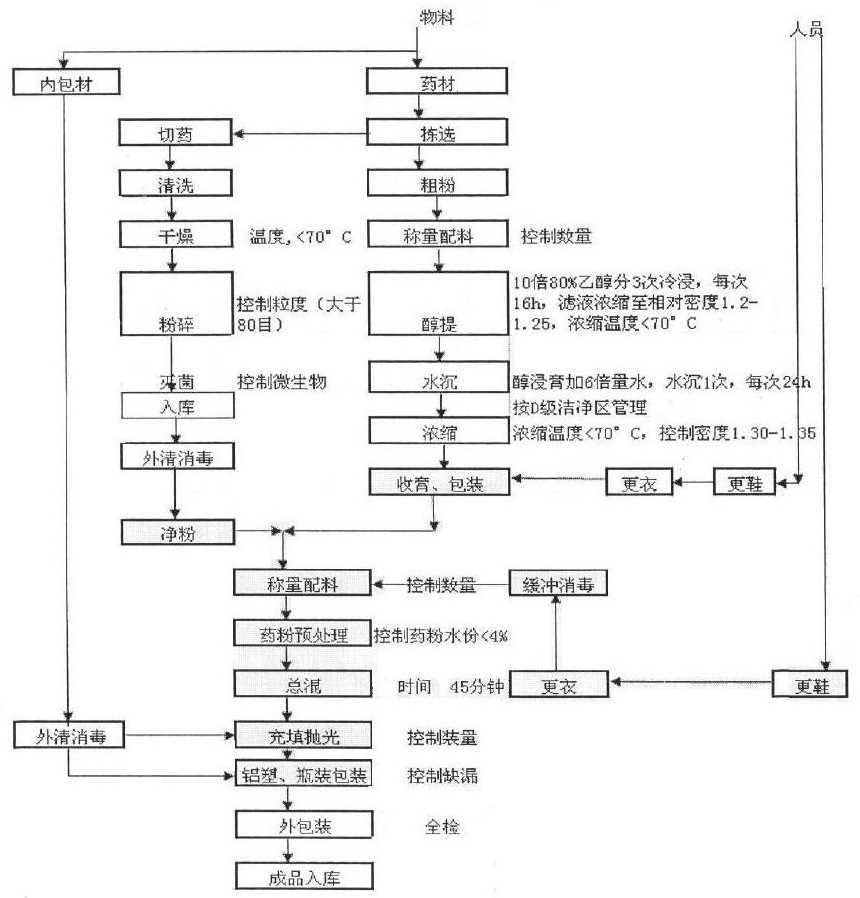
[0093] Prescription: Phyllostachys 5200g
[0094] Preparation method: take 5000 g of Phyllostachys phylloxera coarse powder (40 mesh), and cold soak three times with 10 times the amount of 80% ethanol. (4 times the amount for the first time, 3 times the amount for the second and third times, 16 hours each time) Filtration, the filtrate is controlled at a temperature 80 mesh) crushed in a clean area and qualified for bacterial inspection, mix well, dry at 60°C, crush, sieve, quality inspect, granulate, and fill capsules to make 1000 capsules. . Flowchart such as figure 1 shown.
Embodiment 3
[0095] Embodiment 3: product quality control
[0096] 1) Identification of Phyllostachys phylloxera
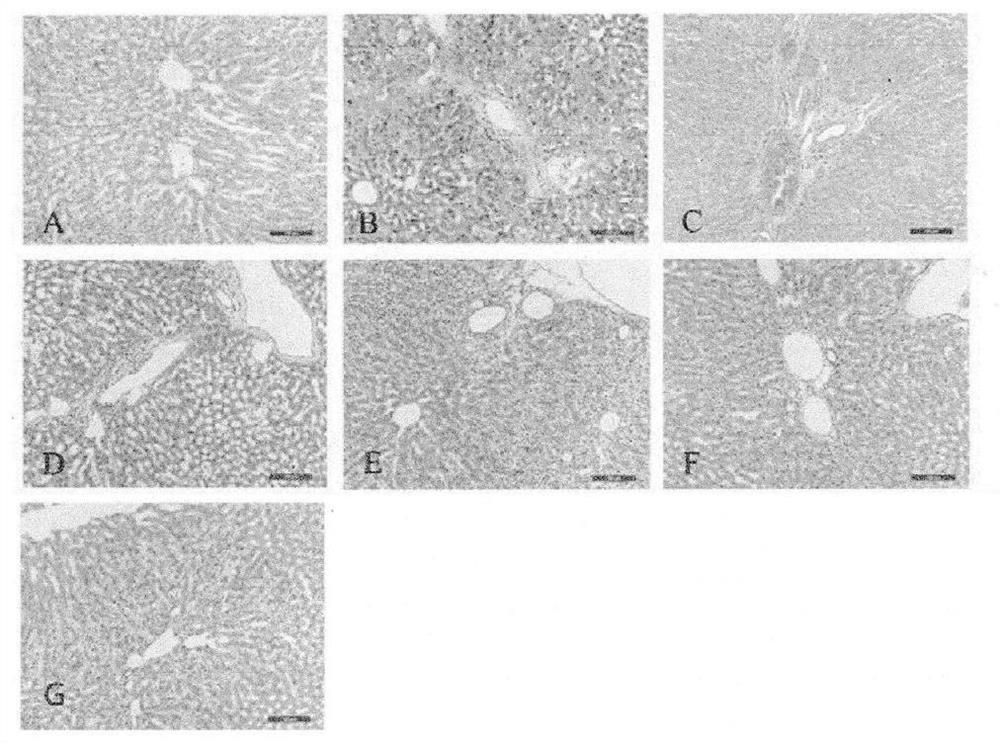
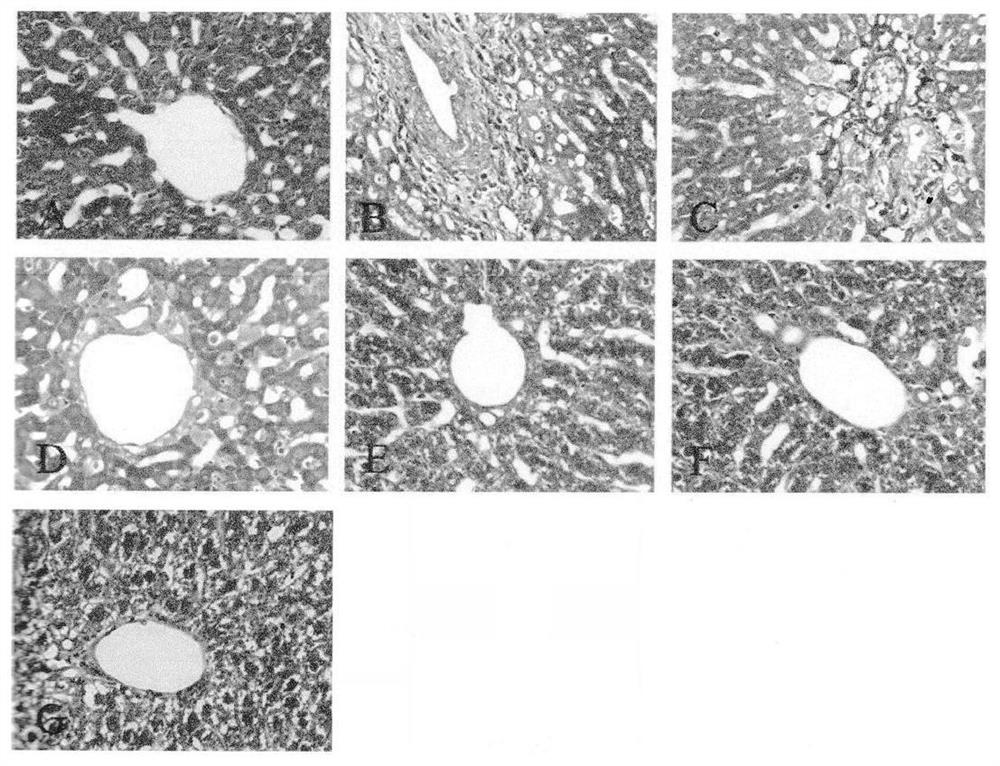
[0097]Take 2g of the content of this product, add 20ml of ethanol, shake for 2 hours, filter, concentrate the filtrate to 2ml, add 2g of silica gel (100-200 mesh), mix well, evaporate the solvent, put it into a chromatographic column, and diethyl ether 8ml for elution, discard the ether solution, add 8ml of ethanol for elution, evaporate to dryness; add 1ml of methanol to dissolve, and use it as the test sample. Another 2 g of Phyllanthus phylloxera reference substance medicinal material was taken, and the reference substance medicinal material solution was prepared in the same way. According to the test of thin-layer chromatography (Appendix VIB of the Chinese Pharmacopoeia 2005 edition), 10 μL of the above two solutions were obtained, and were respectively spotted on the same silica gel G thin-layer plate, with chloroform-methanol (7.5:1) as the developing solvent, Unfold,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com