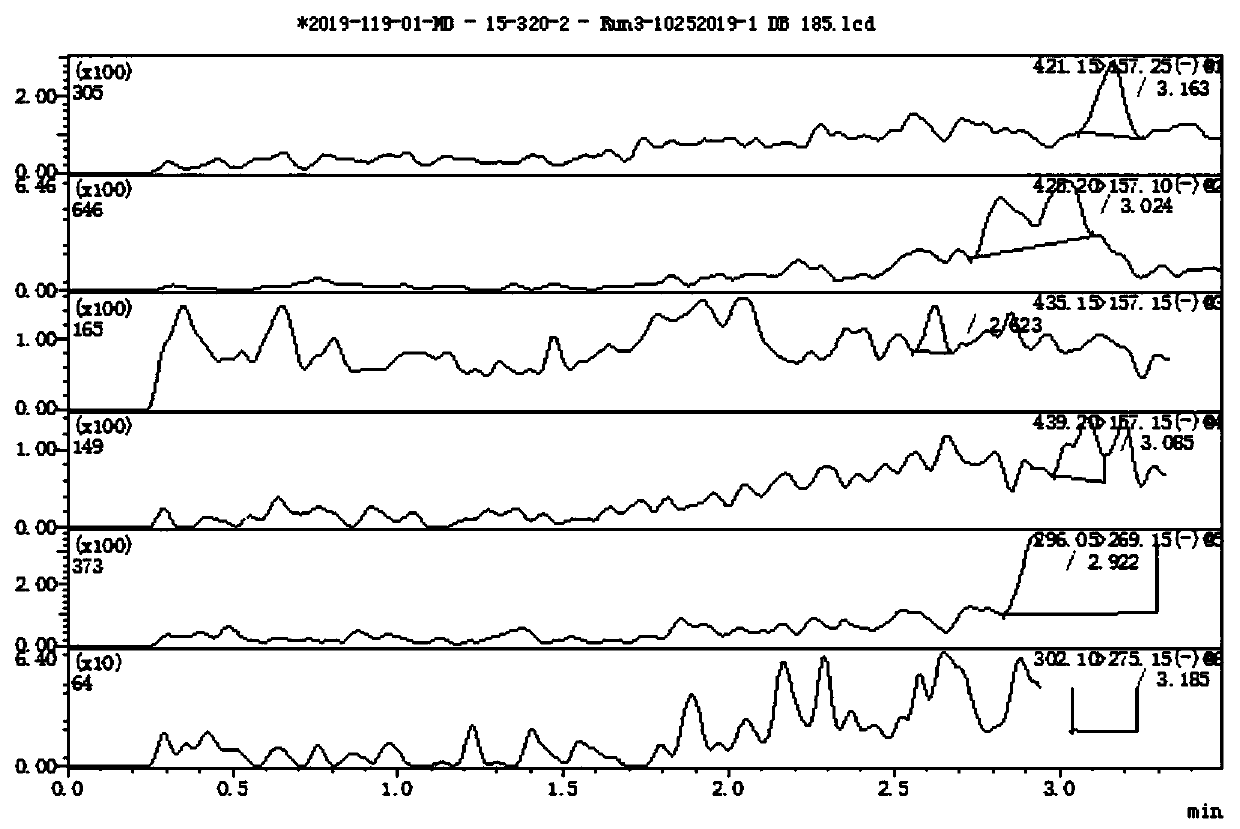
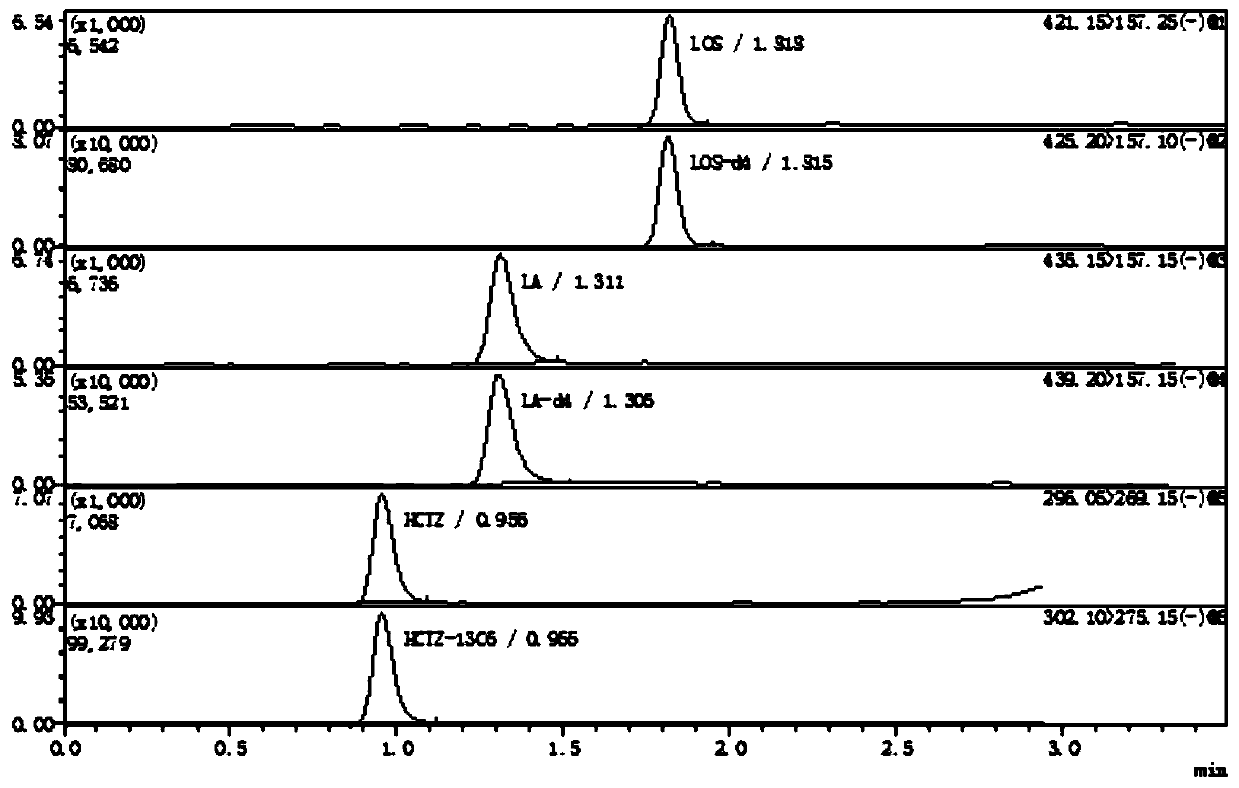
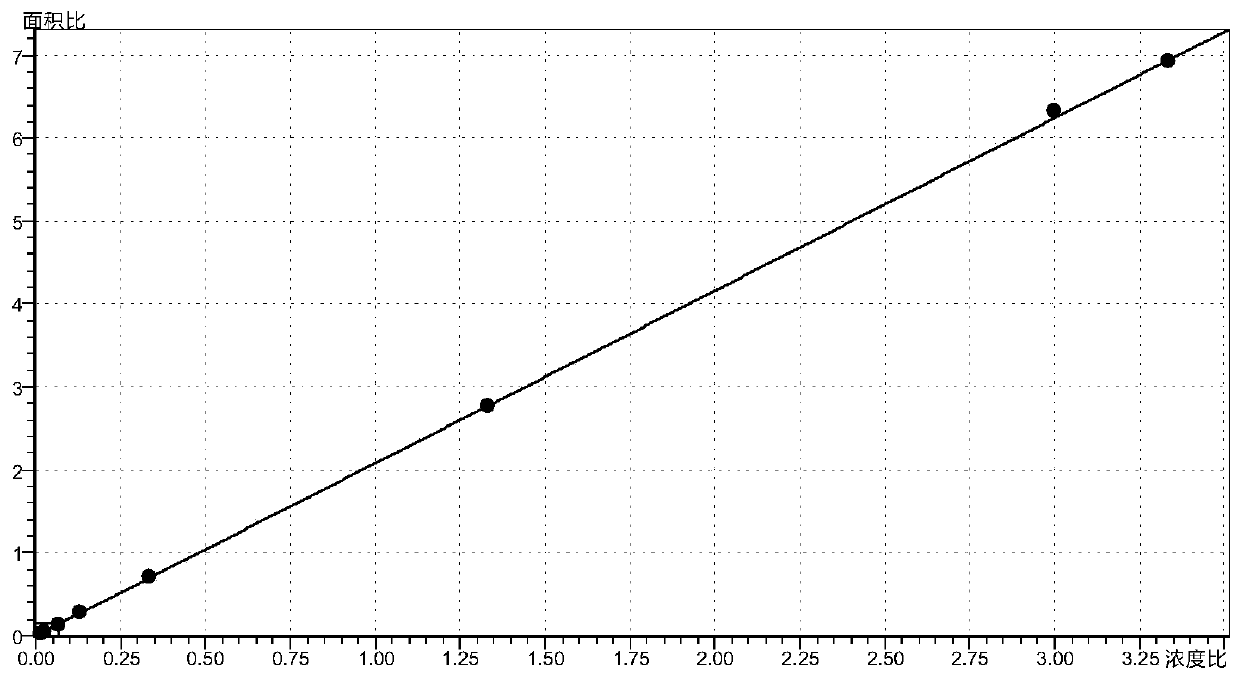
Method for determining concentration of hydrochlorothiazide, losartan and 5-carboxylic acid losartan in plasma by liquid chromatography-mass spectrometry
A technology of hydrochlorothiazide and LC/MS is applied in measurement devices, instruments, scientific instruments, etc., to achieve the effects of simple pretreatment method, fast method and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] 1. Experimental materials and instruments
[0032] Hydrochlorothiazide, losartan and its metabolite 5-carboxylate losartan and various deuterated internal standards hydrochlorothiazide-13C6, losartan-d4 and 5-carboxylate losartan-d4 were provided by TRC in Canada. Acetonitrile, methanol, acetic acid, ammonium acetate, formic acid, etc. are all of HPLC grade, from the American Fisher Company, and other chemical reagents are of analytical grade.
[0033] LCMS-8060 mass spectrometer (Shimadzu, Japan), Lab Solutions 6.84 SP1 data acquisition software (Shimadzu, Japan), Mettler XS 105DU electronic balance (Mettler, Switzerland); Eppendorf Centrifuge5424R high-speed cryogenic centrifuge ( Eppendorf, Germany).
[0034] Second, the experimental process
[0035] 1. Preparation of stock solution and working solution
[0036] Weigh an appropriate amount of hydrochlorothiazide, losartan and its metabolite 5-carboxylate losartan and various deuterated internal standards hydrochlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com