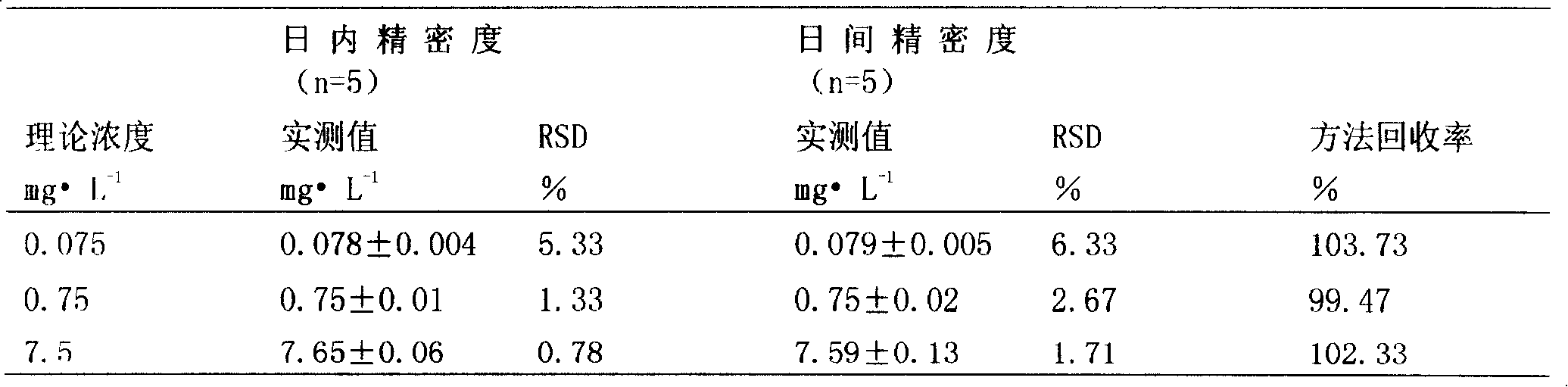
Method for rapidly measuring mizoribine drug concentration
A technology for mizoribine and blood drug concentration, which is applied in the field of medical testing, can solve the problems of measurement accuracy, low precision, tedious and troublesome operation, and high analysis cost, and achieves a low cost, fast and accurate method, and low plasma consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Chromatographic conditions
[0027] Chromatographic column: Hypersil BDS C18 (4.6×250mm, 5μm, Dalian Elite Company); mobile phase: 10mmol L -1 Potassium dihydrogen phosphate solution (containing perchloric acid 10mmol·L -1 , PH6.0 6.5); flow rate: 1.5mL min -1 ; Column temperature: room temperature; Detection wavelength: 280nm.
[0028] Plasma sample pretreatment
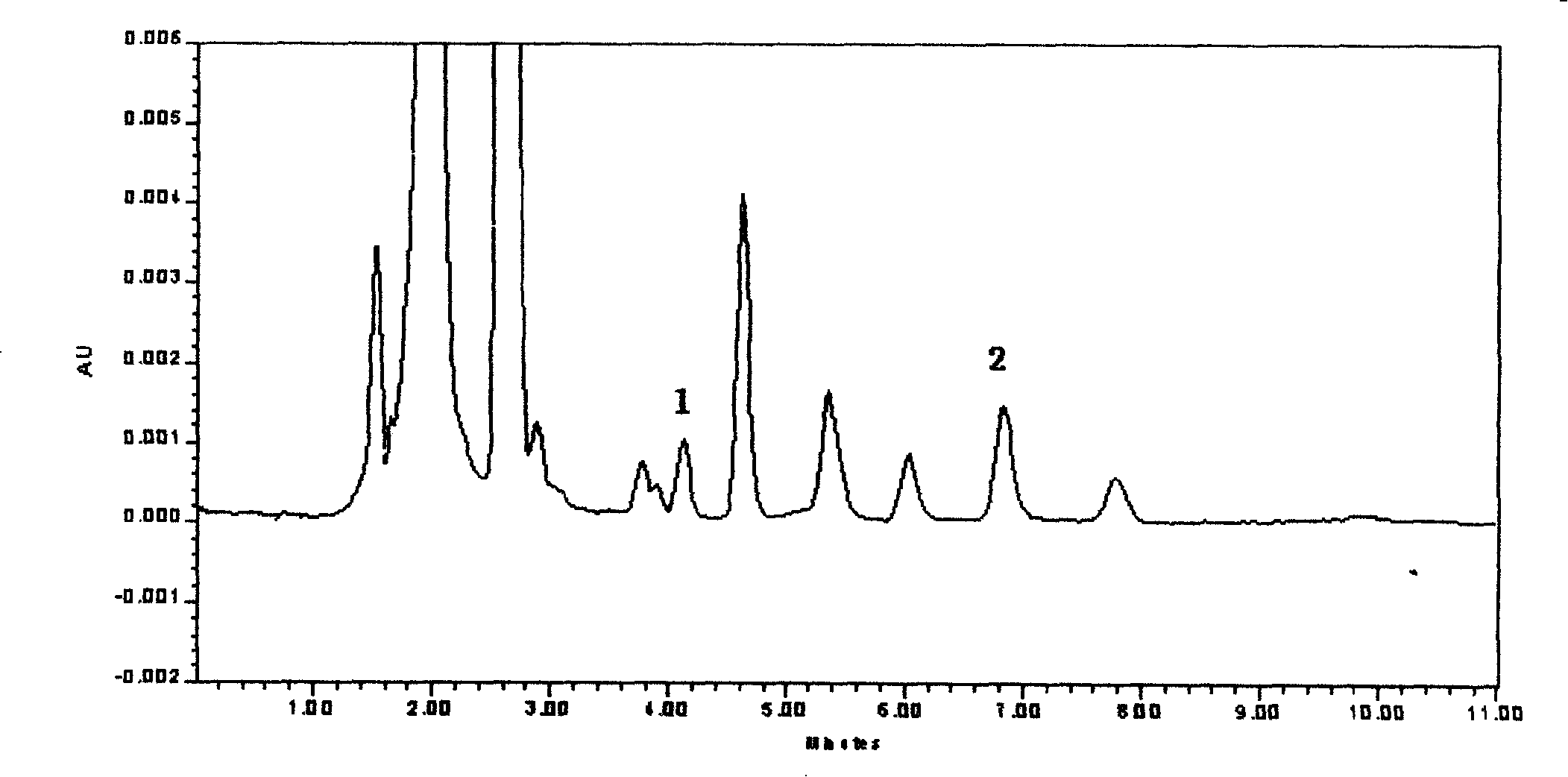
[0029] Take 200 μL of plasma sample, add 20 mg·L -1 Cytarabine 20μL, add 20μL distilled water, vortex mix for 30s, add 0.1mol L -1 Perchloric acid, vortex mixing for 1min, 10000r·min -1 After centrifugation for 5 min, 20 μL of the supernatant was taken for injection. The internal standard method was quantified by the peak area ratio of MZR and cytarabine.
[0030] Exclusive
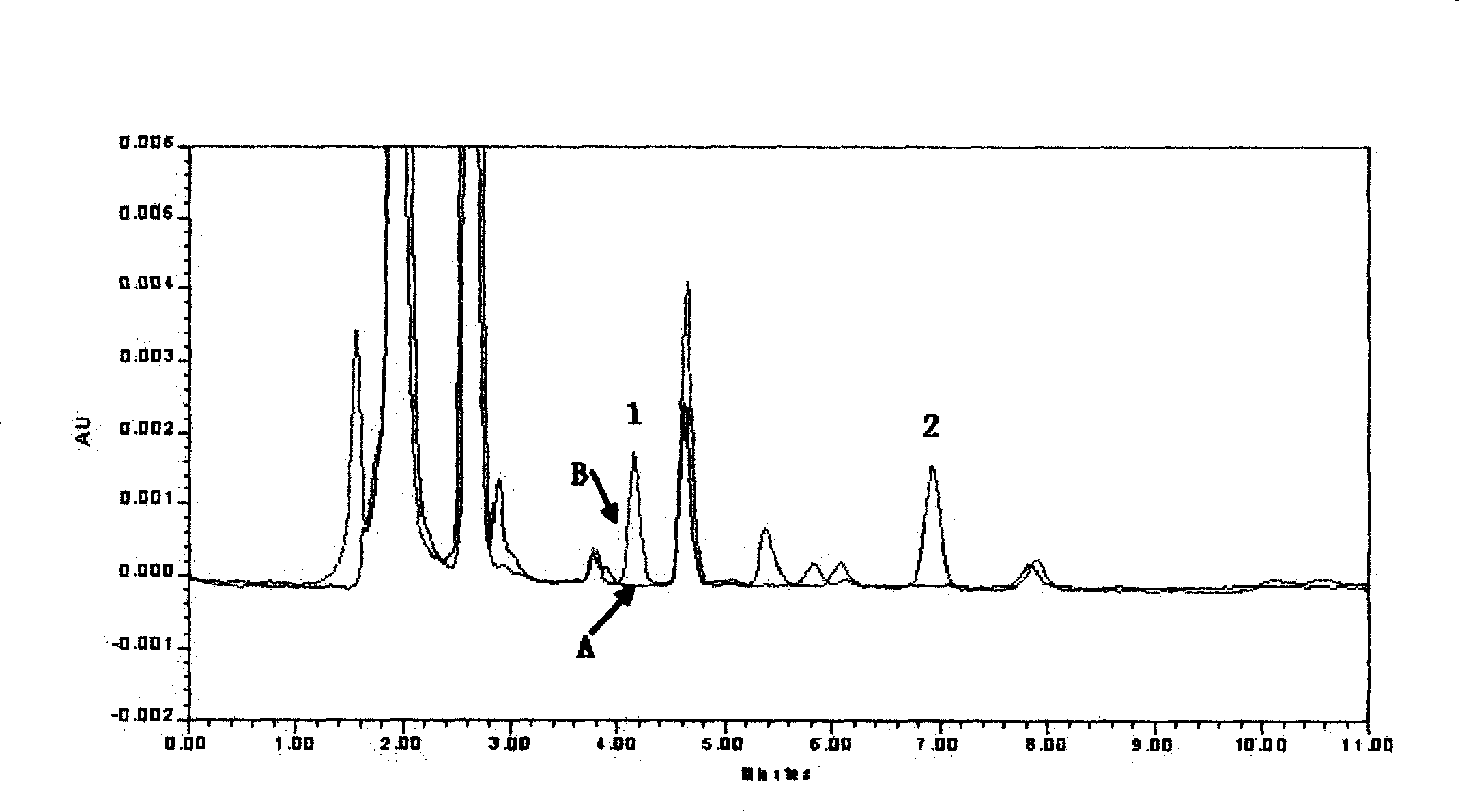
[0031] Blood samples from 10 kidney transplant patients who did not take MZR were taken from different sources, and measured according to the above sample pretreatment and measurement methods. The results showed that no endogenou...
Embodiment 2
[0040] Chromatographic conditions
[0041] Chromatographic column: Hypersil BDS C18 (4.6×250mm, 5μm, Dalian Elite Company); mobile phase: 10mmol L -1 Potassium dihydrogen phosphate solution (containing 20mmol·L perchloric acid -1 , PH6.0-6.5); flow rate: 1.5mL min -1 ; Column temperature: room temperature; Detection wavelength: 280nm.
[0042] Plasma sample pretreatment
[0043] Take 200 μL of plasma sample, add 20 mg·L -1 Cytarabine 20 μL, add 20 μL distilled water, vortex mix for 30 seconds, add 0.25mol L -1 Perchloric acid, vortex mixing for 1min, 10000r·min -1 After centrifugation for 5 min, 20 μL of the supernatant was taken for injection. The internal standard method was quantified by the peak area ratio of MZR and cytarabine.
[0044] Exclusive
[0045] Blood samples from 10 kidney transplant patients who did not take MZR were taken from different sources, and measured according to the above sample pretreatment and measurement methods. The results showed that no...
Embodiment 3
[0051] Chromatographic conditions
[0052] Chromatographic column: Hypersil BDS C18 (4.6×250mm, 5μm, Dalian Elite Company); mobile phase: 10mmol L -1 Potassium dihydrogen phosphate solution (containing 30mmol·L perchloric acid -1 , PH6.0-6.5); flow rate: 1.5mL min -1 ; Column temperature: room temperature; Detection wavelength: 280nm.
[0053] Plasma sample pretreatment
[0054] Take 200 μL of plasma sample, add 20 mg·L -1 Cytarabine 20 μL, add 20 μL distilled water, vortex mix for 30 seconds, add 0.5mol L -1 Perchloric acid, vortex mixing for 1min, 10000r·min -1 After centrifugation for 5 min, 20 μL of the supernatant was taken for injection. The internal standard method was quantified by the peak area ratio of MZR and cytarabine.
[0055] Exclusive
[0056] Blood samples from 10 kidney transplant patients who did not take MZR were taken from different sources, and measured according to the above sample pretreatment and measurement methods. The results showed that no ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com