A method for separating and determining degraded impurities in dutasteride raw materials and preparations by hplc method
A technology of dutasteride and API, applied in the field of analytical chemistry, can solve problems such as obvious interference, and achieve the effect of good specificity, high accuracy and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Equipment and chromatographic conditions:
[0036] Japan Shimadzu SHIMADZULC-2010AHT liquid chromatograph; Detector: UV; Chromatographic column: octadecylsilane bonded silica gel is the chromatographic column (Kromasil 100-5C18, 250mm * 4.6mm, 5 μ m) of filler; With acetonitrile- Water-trifluoroacetic acid (520:480:0.25) was mobile phase A, methanol was mobile phase B, the flow rate was 1.0ml / min, the column temperature was 35°C, the detection wavelength was 310nm, and the injection volume was 50μl.
[0037] Gradient elution conditions:
[0038]
[0039] Detection steps:
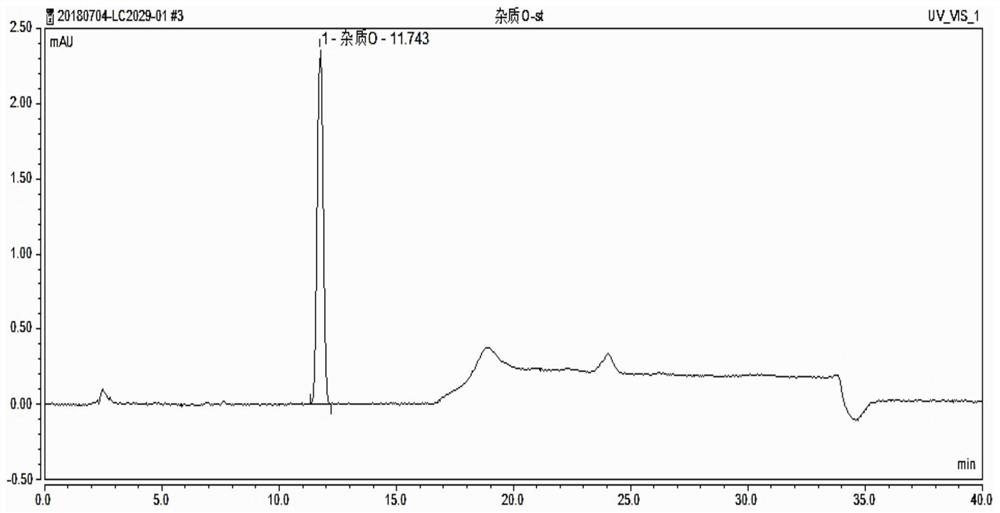
[0040] 1. Preparation of the test solution: take 10 dutasteride soft capsules, pierce the top of the capsule with scissors, squeeze the contents into a 25ml measuring bottle, and use 70% acetonitrile aqueous solution to divide the scissors and the contents on the capsule shell After washing, the washing liquid was combined in the above-mentioned measuring bottle, diluted to the mark with 70% acet...
Embodiment 2
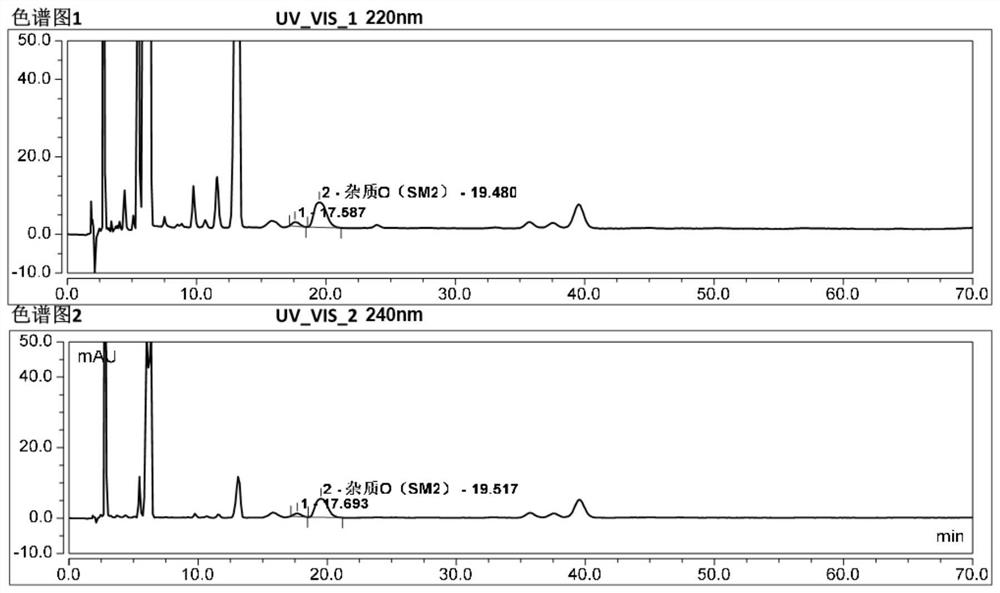
[0049] Embodiment 2 explores the conditions of the detection wavelength when detecting 2,5-bistrifluoromethylaniline.
[0050] Detection method:
[0051] Use octadecylsilane bonded silica gel as a chromatographic column (Kromasil 100-5C18, 250mm×4.6mm, 5μm, or a chromatographic column with equivalent performance); use acetonitrile-water-trifluoroacetic acid as mobile phase A, and use Methanol was the mobile phase B; the flow rate was 1ml / min; the column temperature was 35°C; the detection wavelengths were 220nm and 240nm.
[0052] Test results:
[0053] like figure 2 shown. According to the ultraviolet absorption spectrum, it is found that 2,5-bistrifluoromethylaniline has the maximum absorption near 240nm and 310nm. 240nm was used as the detection wavelength, and the blank excipients interfered with the detection. Therefore, the detection wavelength was initially selected as 310nm. In addition, compared with 220nm and 240nm, at 310nm wavelength, other known impurities a...
Embodiment 3
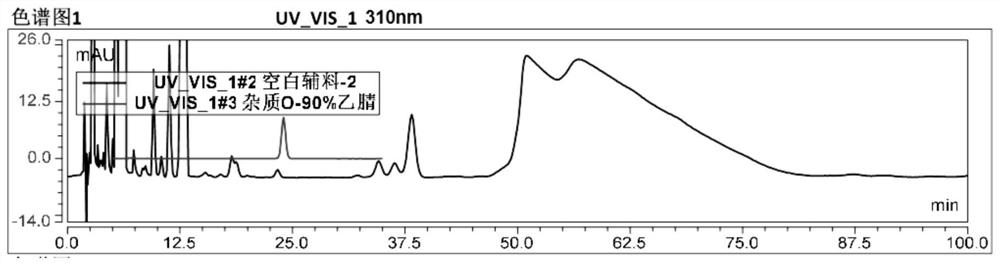
[0055] Embodiment 3 explores the conditions of diluent concentration when detecting 2,5-bistrifluoromethylaniline.
[0056] Detection method:
[0057] A chromatographic column (Kromasil 100-5C18, 250mm×4.6mm, 5μm, or a chromatographic column with equivalent performance) with octadecylsilane bonded silica gel as a filler, and acetonitrile-water-trifluoroacetic acid (520:480:0.25 ) is the mobile phase A, methanol is the mobile phase B; the flow rate is 1ml / min, the column temperature is 35°C, and the detection wavelength is 310nm.
[0058] Diluent: screen acetonitrile, 90% acetonitrile in water, 80% acetonitrile in water, 70% acetonitrile in water, 60% acetonitrile in water, 50% acetonitrile in water.
[0059] Detection steps: dilute the test sample with diluent solvents of different concentrations, and inject samples respectively according to the chromatographic method of the present invention.
[0060] Test results:
[0061] like image 3 shown. 1) Using 60% acetonitrile,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com