A method for measuring the concentration of sodium tanshinone IIA sulfonate in human plasma
A technology of tanshinone and sodium sulfonate, which is applied in the field of medical testing, can solve the problems of low sensitivity that cannot meet measurement requirements, specificity cannot meet measurement requirements, and inaccurate measurement results, and achieves low cost, stable recovery, and plasma dosage. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Chromatographic conditions: Japan Shimadzu UFLC system: SIL-30AC autosampler (1 set), LC-30AD infusion pump (2 sets), DGU-20A 5R Online degasser (1 set), CBM-20A controller (1 set), CTO-30A column thermostat (1 set), data acquisition and processing software from AB Sciex, USA: Analyst 1.6.1. XSELECTTM HSS T3 of American Waters Company, 2.1 × 100mm, 3.5 μ m, column temperature: room temperature, mobile phase: aqueous solution containing 0.45mmol / L ammonium formate and 18ppm formic acid: acetonitrile (40:60, V / V); flow rate 0.3mL min -1 ;
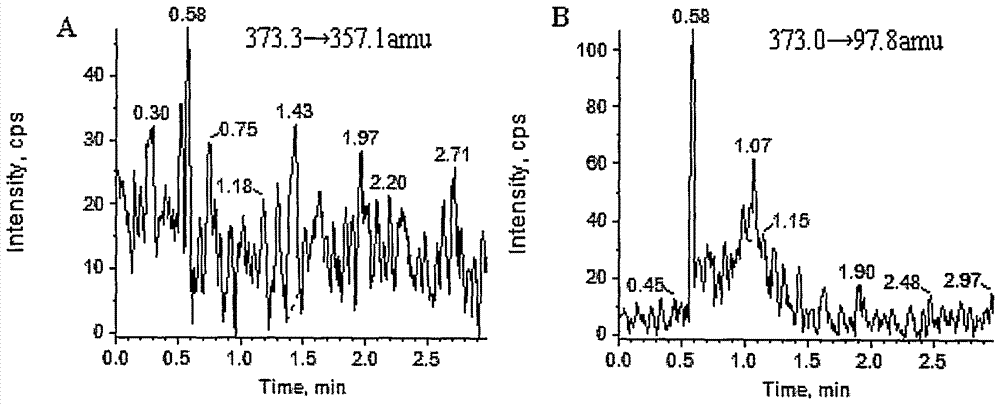
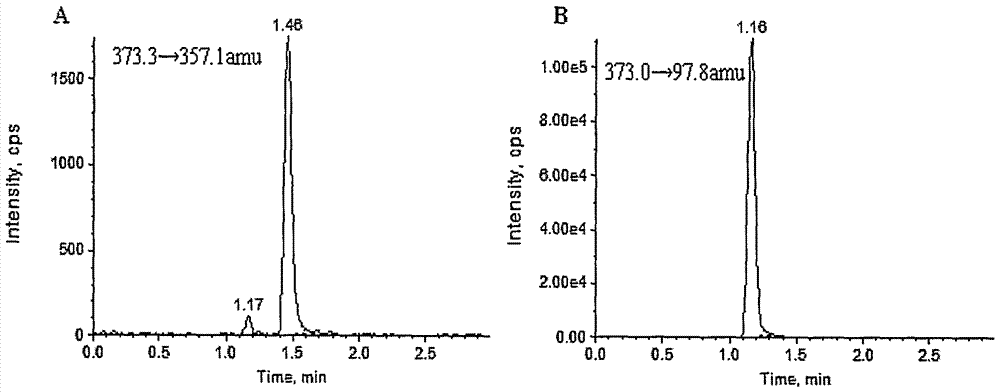
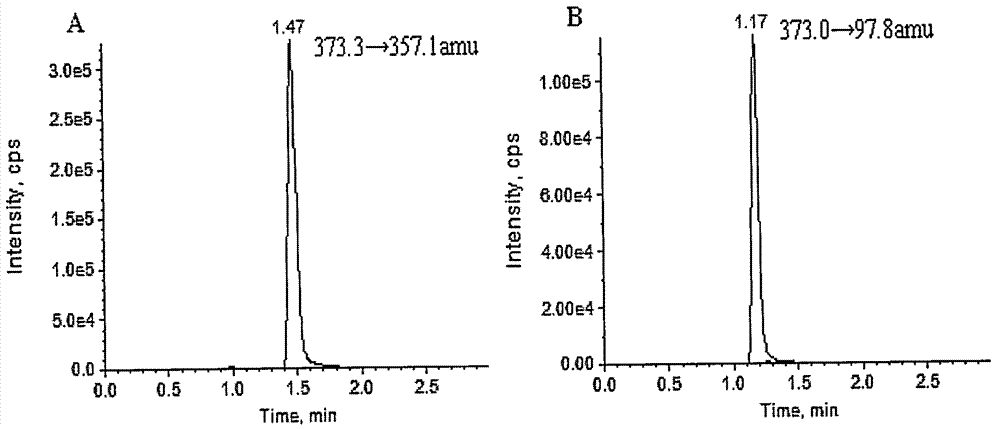
[0039] Mass Spectrometry Conditions: Triple Quad, AB Sciex, USA TM5500 tandem mass spectrometer, mass spectrometer ionization method: electrospray ion source. Ion source parameters: GS1: 60psi; GS2: 60psi; curtain gas: 25psi; spray voltage: -4500V; Collision energy of D5: 50±10eV; ion channel selection: STS: 373.3→357.1amu, DHEAS-D5: 373.0→97.8amu; scanning interval: 5ms;
[0040] Plasma sample pretreatment
[0041] Take 0.1 mL of...
Embodiment 2
[0055] Chromatographic conditions
[0056] Japan Shimadzu UFLC system: SIL-30AC autosampler (1 set), LC-30AD infusion pump (2 sets), DGU-20A 5R Online degasser (1 set), CBM-20A controller (1 set), CTO-30A column thermostat (1 set), data acquisition and processing software from AB Sciex, USA: Analyst 1.6.1. XSELECTTM HSS T3 from American Waters Company, 2.1×100mm, 3.5μm, column temperature: room temperature. Mobile phase: aqueous solution containing 0.40 mmol / L ammonium formate and 16 ppm formic acid: acetonitrile (45:55, V / V); flow rate 0.3 mL min -1 ;
[0057] Mass Spectrometry Conditions:
[0058] Triple Quad of AB Sciex, USA TM 5500 tandem mass spectrometer, mass spectrometer ionization method: electrospray ion source, ion source parameters: GS1: 60psi; GS2: 60psi; curtain gas: 25psi; spray voltage: -4500V; capillary temperature: 550°C; collision gas: nitrogen, scan mode : Multiple reaction ion detection scan (MRM); Collision energy of STS and DHEAS-D5: 50±10eV; Ion c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com