A kind of assay method of residual solvent in decitabine intermediate
A residual solvent and determination method technology, applied in the field of pharmaceutical analysis, can solve the problems of poor stability of raw glycosides, solvent peak interference, low recovery rate, etc., and achieve the effects of good method recovery rate, high accuracy and high recovery rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
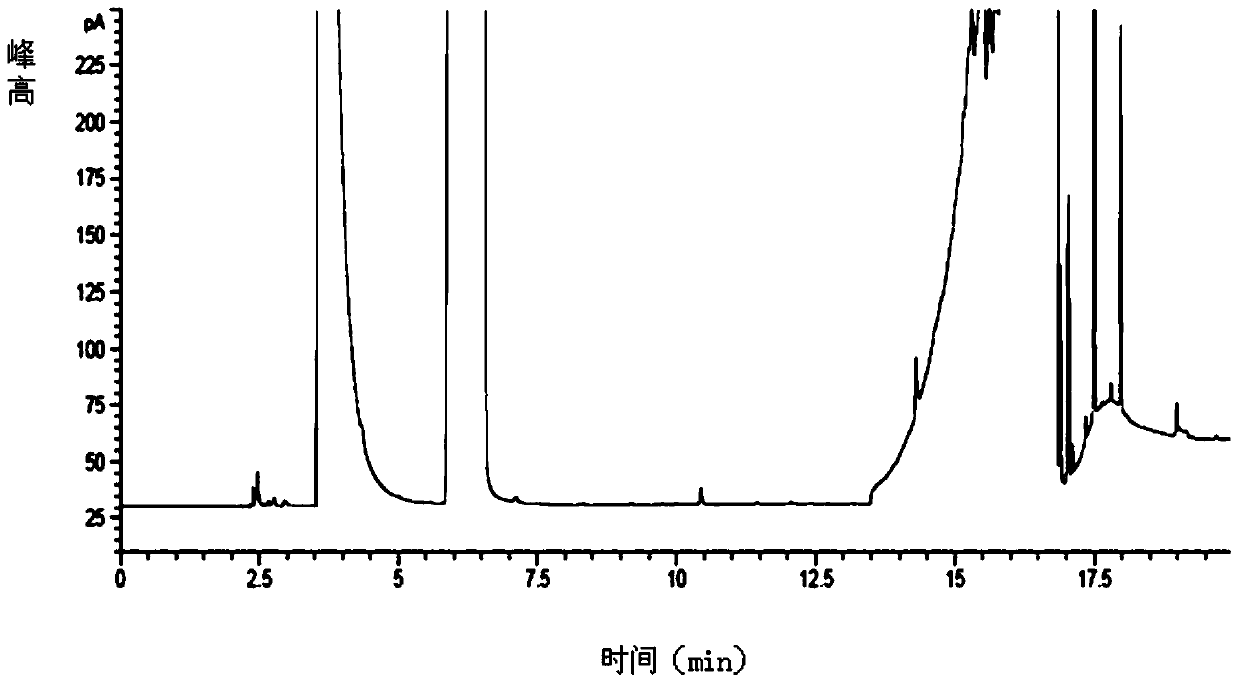
Embodiment 1
[0072] Chromatographic conditions:
[0073] Column temperature: programmed temperature rise: the initial temperature was 40°C and kept for 8 minutes, then the temperature was raised to 220°C at a rate of 20°C per minute and kept for 19 minutes.
[0074] Injection port temperature: 250°C;
[0075] Flame ionization detector temperature: 300°C;
[0076] Carrier gas: nitrogen;
[0077] Carrier gas flow rate: 3.0ml / min;
[0078] Split ratio: 20:1.
[0079] 1. Detection
[0080] A. Preparation of the test solution:
[0081] First, prepare a mixed solution of methanol-N-methylpyrrolidone-triethylamine in a volume ratio of 47.5:47.5:5 and set aside;
[0082] Again, accurately weigh 1.0 g of glucoside, put it in a 10 ml volumetric flask, dissolve and dilute it into a 0.1 g / ml solution with the prepared mixed solution of methanol-N-methylpyrrolidone-triethylamine, mix well, and set aside;
[0083] B. Preparation of reference substance solution: Accurately weigh 200 μg of pyridine...
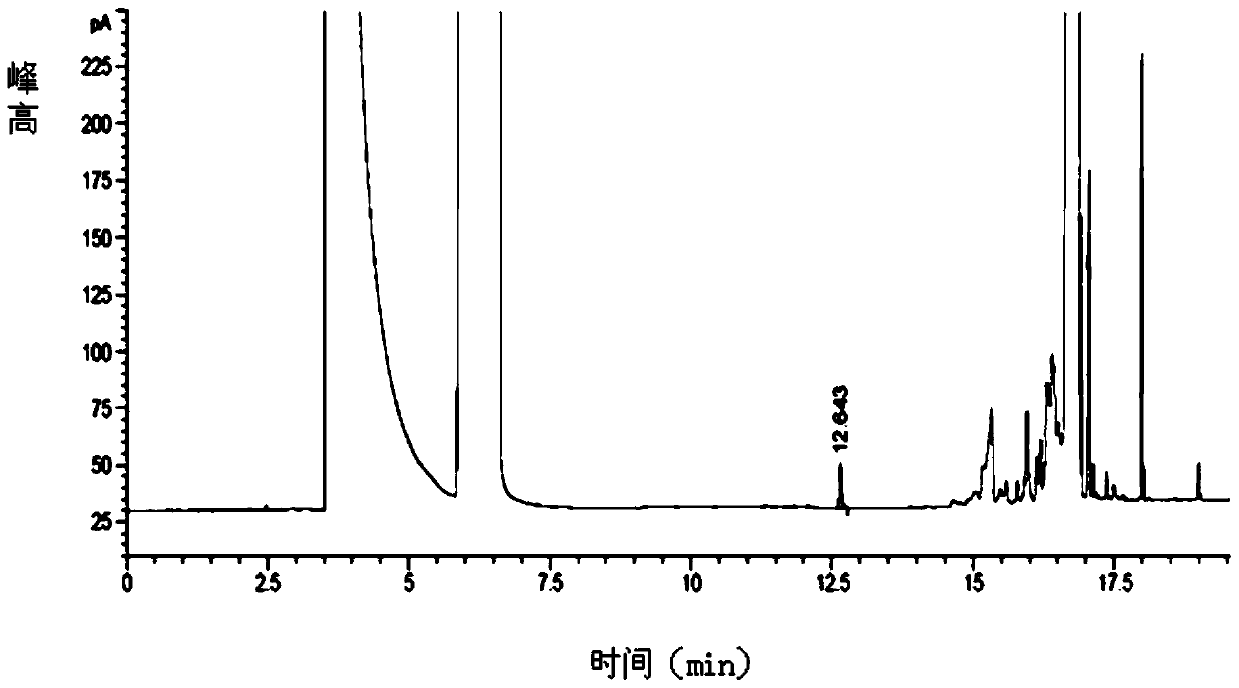
Embodiment 2
[0127] The difference between this embodiment and embodiment 1 is that the volume ratio of methanol-N-methylpyrrolidone-triethylamine is
[0128] 45:45:10.
[0129] Chromatographic conditions:
[0130] Column temperature: programmed temperature rise: the initial temperature was 40°C and kept for 8 minutes, then the temperature was raised to 220°C at a rate of 20°C per minute and kept for 19 minutes.
[0131] Injection port temperature: 230°C;
[0132] Flame ionization detector temperature: 280°C;
[0133] Carrier gas: nitrogen;
[0134] Carrier gas flow rate: 2.0ml / min;
[0135] Split ratio: 10:1.
[0136] According to the above conditions, three batches of test solution were detected, and no pyridine peak was detected in the chromatograms. After calculation, the residual amount of pyridine in the glycoside was 0%.
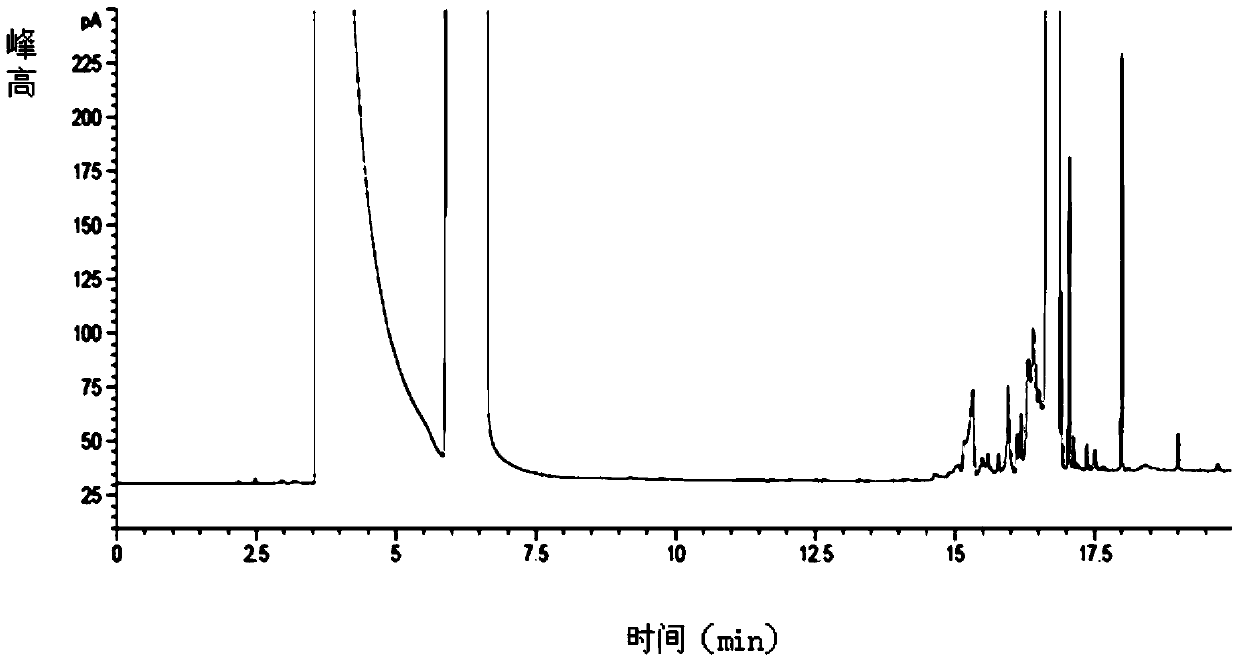
Embodiment 3
[0138] The difference between this embodiment and embodiment 1 is that the volume ratio of methanol-N-methylpyrrolidone-triethylamine is
[0139] 48:48:4.
[0140] Chromatographic conditions:
[0141] Column temperature: programmed temperature rise: the initial temperature was 40°C and kept for 8 minutes, then the temperature was raised to 220°C at a rate of 20°C per minute and kept for 19 minutes.
[0142] Injection port temperature: 270°C;
[0143] Flame ionization detector temperature: 320°C;
[0144] Carrier gas: nitrogen;
[0145] Carrier gas flow rate: 4.0ml / min;
[0146] Split ratio: 30:1.
[0147] According to the above conditions, three batches of test solution were detected, and no pyridine peak was detected in the chromatograms. After calculation, the residual amount of pyridine in the glycoside was 0%.
[0148] In addition, other residual solvents in the test product can also be detected according to the methods provided in the above-mentioned Examples 1 to 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com