Method for determining human plasma phenytoin and its precursor drug and metabolite
A prodrug, phenytoin technology, applied in the field of medical testing, to achieve the effect of fast and accurate method, strong selectivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Chromatographic conditions
[0032] HPLC system: column Agilent RX-C 8 (250mm×4.6mm, 5μm), the mobile phase is 0.08% TFA aqueous solution-acetonitrile-methanol (60:17:23, V / V / V), flow rate: 1.5mL min -1 ; Column temperature: 40°C; UV detection wavelength: 210±1nm.
[0033] Plasma sample pretreatment
[0034] Precisely draw 100 μL of plasma sample into a 1.5 mL centrifuge tube, add 50 μL of 20% phosphoric acid for acidification, vortex for 0.5 min, add 1 mL containing 10 mg·L -1 Extract propranolol hydrochloride with ethyl acetate solution, vortex for 5min, centrifuge (15,000×g, 4°C) for 10min, take 800μL of the upper layer extract, transfer to a 5mL centrifuge tube, place in a water bath at 40°C, blow dry with nitrogen , add 100 μL of mobile phase, take 20 μL of supernatant for injection analysis, and quantify by peak area by internal standard method.
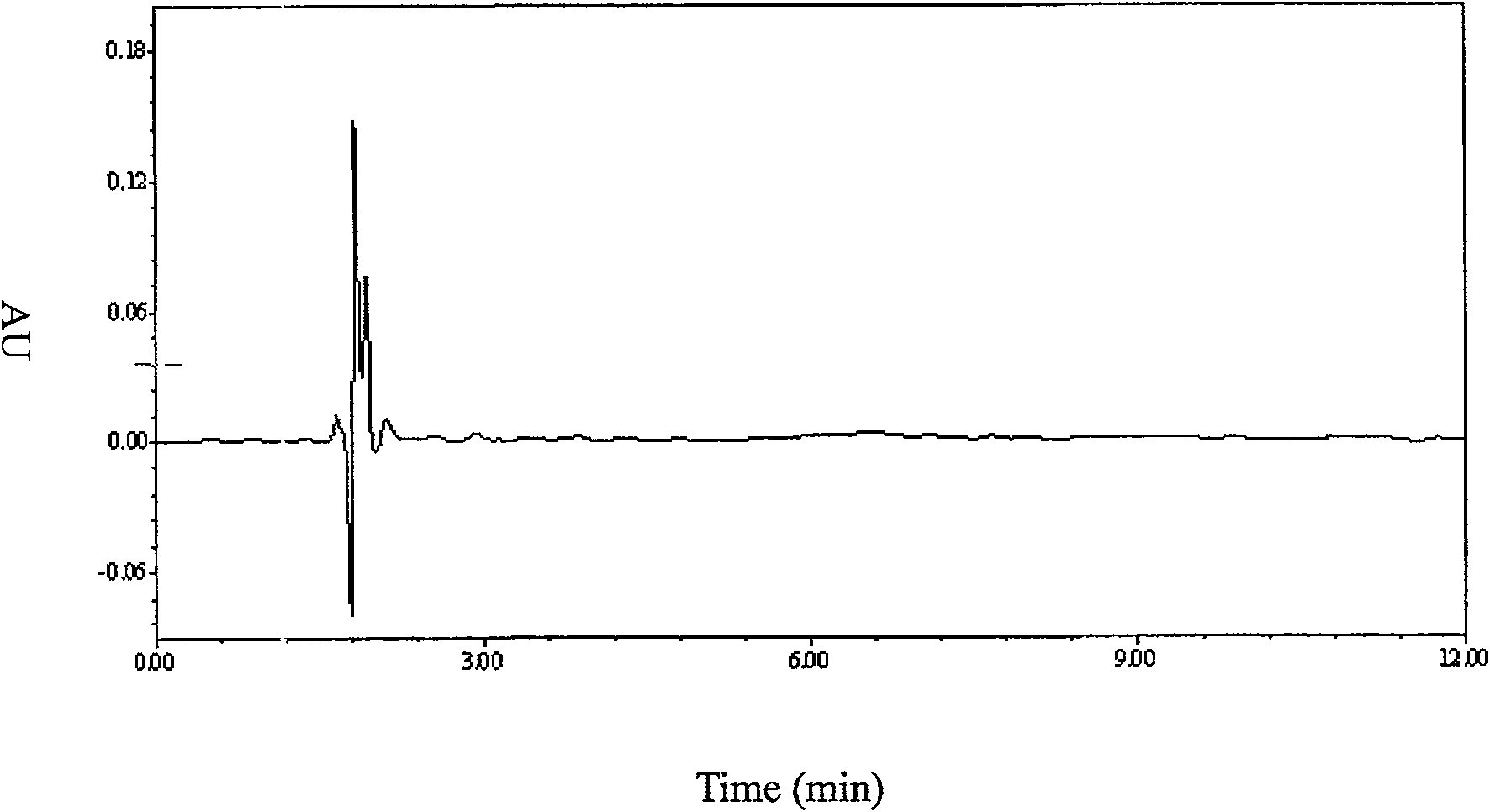
[0035] Exclusive
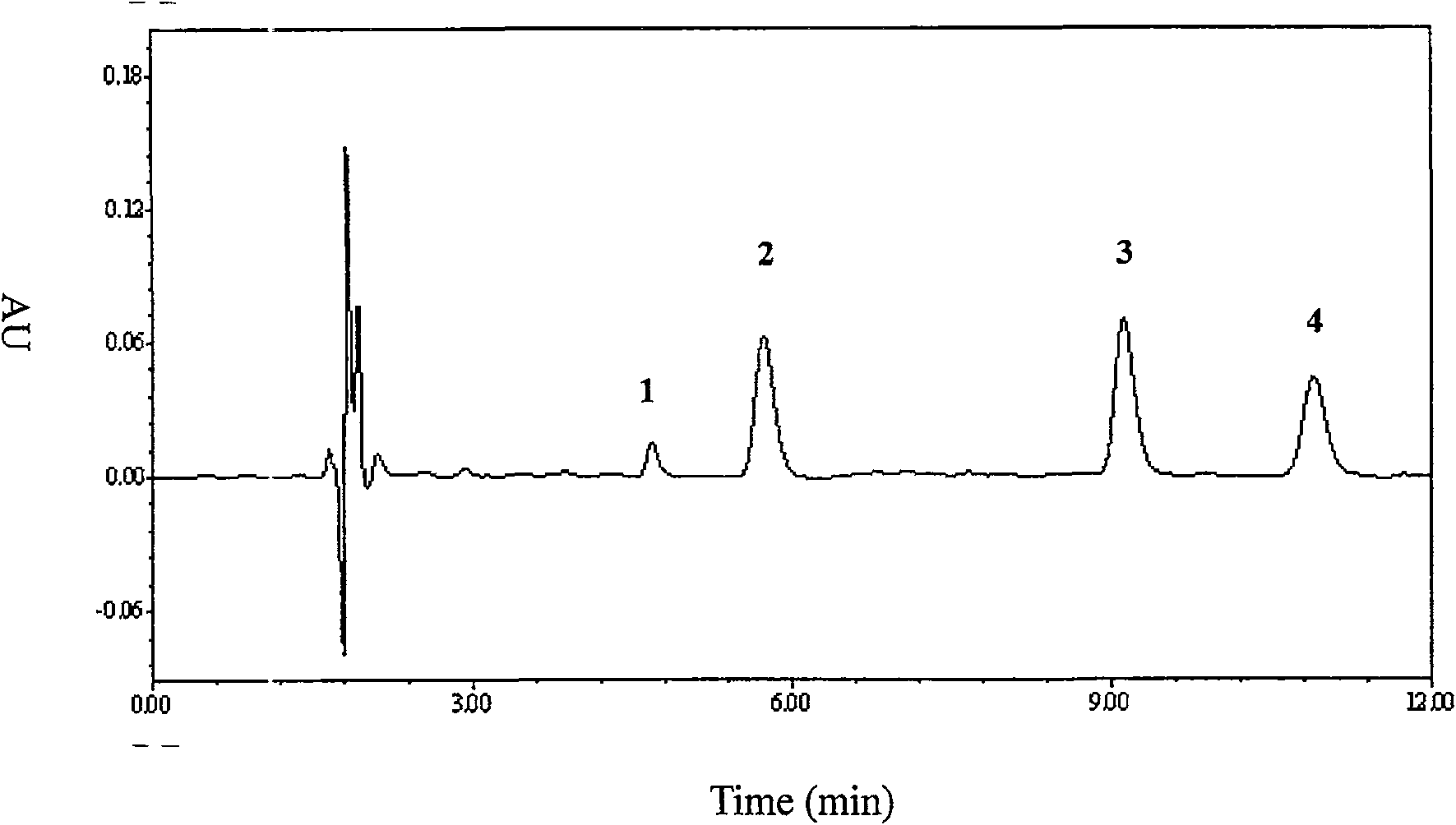
[0036] The blank plasma of 10 subjects who did not take fosphenytoin, phenytoin and 4′-hydroxy...
Embodiment 2
[0044] Chromatographic conditions
[0045] HPLC system: column Agilent RX-C 8 (250mm×4.6mm, 5μm), the mobile phase is 0.1% TFA aqueous solution-acetonitrile-methanol (60:17:23, V / V), flow rate: 1.5mL min -1 ;Column temperature: 40°C; UV detection wavelength: 210±1nm; Injection volume: 20μL.
[0046] Plasma sample pretreatment
[0047] Precisely draw 100 μL of plasma sample into a 5 mL centrifuge tube, add 25 μL of 40% phosphoric acid to acidify, vortex for 0.5 min, add 2 mL containing 10 mg L -1 Extract propranolol hydrochloride with ethyl acetate solution, vortex for 5min, centrifuge at 15,000×g, 4°C) for 10min, take 1.8mL of the upper layer extract, transfer it to a 5mL centrifuge tube, put it in a water bath at 40°C, and blow it with nitrogen , add 100 μL of mobile phase, vortex and redissolve, take 20 μL of the supernatant for sample analysis, and quantify by peak area by internal standard method.
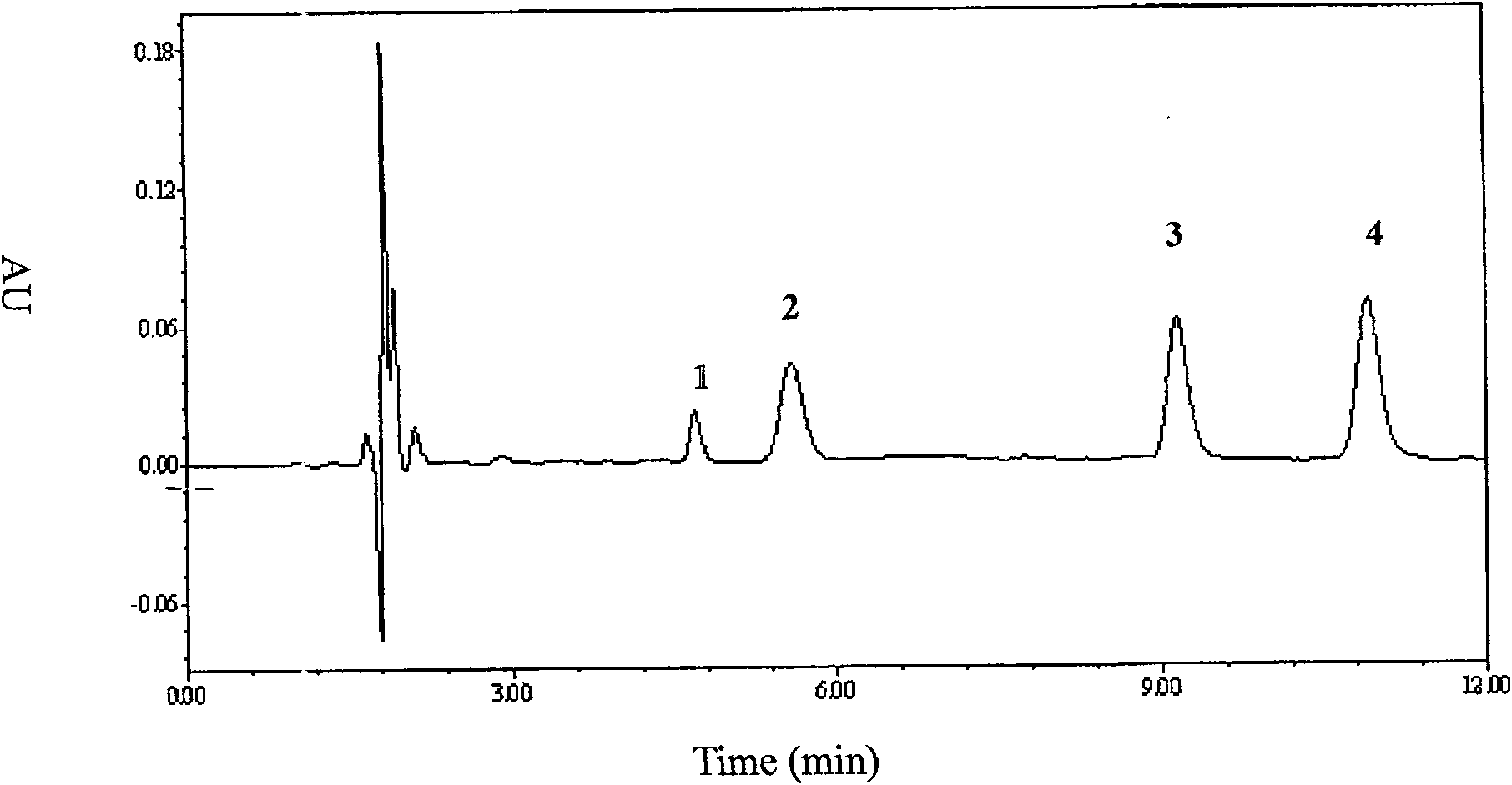
[0048] Exclusive
[0049] The blank plasma of 10 subjects who did not t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com