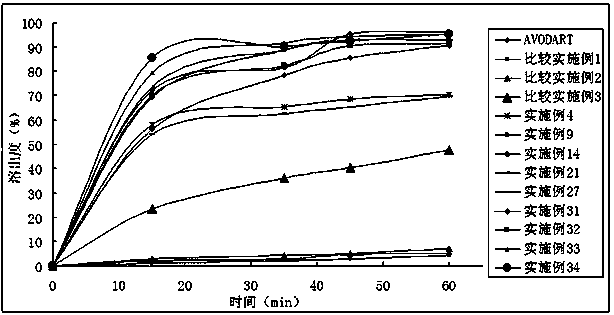
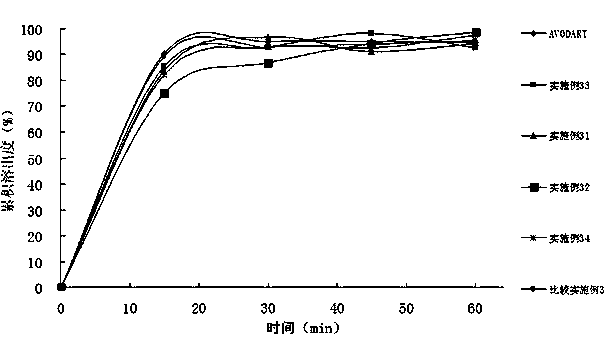
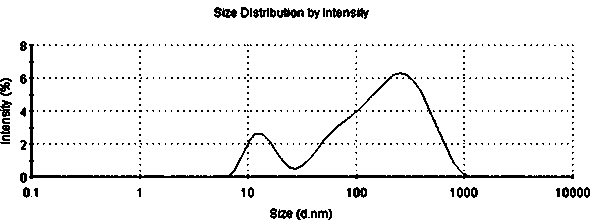
Dutasteride self-microemulsion composition and preparation method thereof
A technology of dutasteride and composition, applied in the field of pharmaceutical preparations, can solve problems such as being unfavorable to control the particle size of emulsion droplets, complicated preparation process, etc., and achieve the effects of improving dissolution rate and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1-30 Self-microemulsion composition
[0072]
[0073]
[0074] The above-mentioned dutasteride self-microemulsion capsules (containing 0.5 mg of dutasteride / capsule) can be prepared by the following process:
[0075] Process Ⅰ: Accurately weigh the prescribed amount of dutasteride, add the prescribed amount of co-emulsifier, dissolve it in a water bath at 40°C, add the oil phase and emulsifier according to the prescribed amount, stir evenly to obtain dutasteride self-microemulsion, pour it into Dutasteride self-microemulsion hard capsules were obtained by filling them in hard capsules.
[0076] Process Ⅱ: Accurately weigh the prescription amount of oil phase, emulsifier and co-emulsifier, stir evenly to prepare a blank self-microemulsion concentrate, then weigh the prescription amount of dutasteride, stir to dissolve and mix evenly to obtain dutasteride Dutasteride hard capsules are prepared by self-microemulsion filling in hard capsules.
[0077] Pr...
Embodiment 19
[0081] Dutasteride self-microemulsion 7g of Example 19 (containing 21mg of dutasteride)
[0082] Soluble starch 35g
[0083] Preparation method: 35 g of soluble starch was added to 7 g of dutasteride self-microemulsion (containing 21 mg of dutasteride) of Example 19 under stirring, and after mixing, capsules were filled according to the content, and a total of 42 solid oral capsules were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com