Production process of high-purity dutasteride
A dutasteride, high-purity technology, applied in the field of pharmaceutical synthesis, can solve the problems of low product yield and purity, complicated purification steps, and high purification costs, and achieves improved purity, improved yield and purity, and improved product quality. The effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
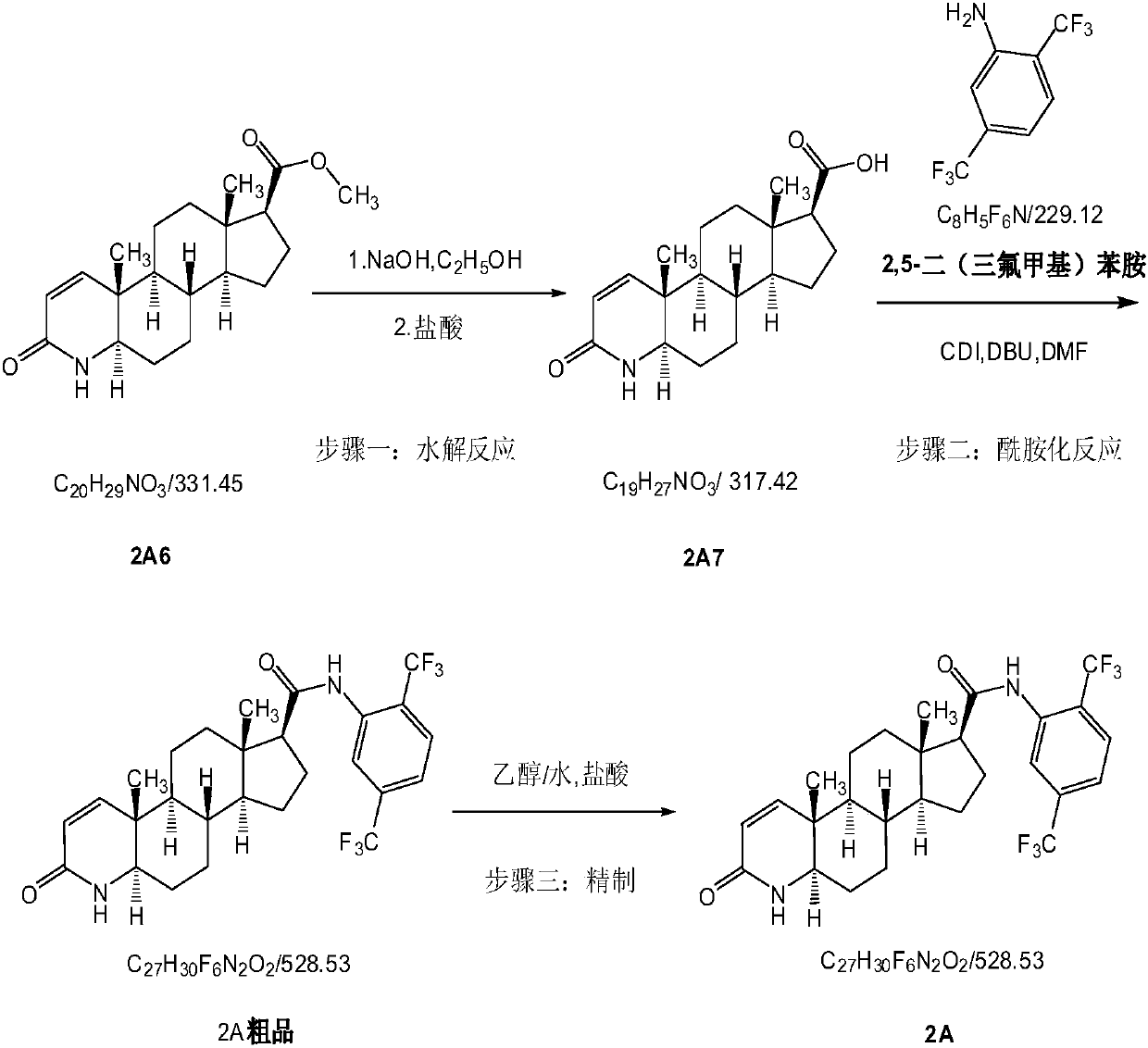
[0052] 1) Add ethanol, 2A6, water, and NaOH (molar ratio: 4:1:9:2) to the reaction kettle in sequence, heat to 75-85°C for 6-8 hours, and TLC detects that the raw material 2A6 is completely reacted ( Developing agent: EA:PE=1:1, UV254nm, product Rf=0). When the temperature is lowered to 60-70°C, add hydrochloric acid to adjust the pH to about 0.8-1.5, and solids are precipitated. Continue to cool down to 20-25°C and stir for 30-45 minutes. °C and dried under reduced pressure for 6-8 hours to obtain off-white powder 2A7.
[0053] 2) Add DMF, 2,5-bis(trifluoromethyl)aniline, DBU, CDI (molar ratio: 5:1:1.3) to the reaction kettle in turn under stirring, and heat to 105-110°C for 2.5-3 After 1 hour, add 2A7 and DMF (molar ratio: 1.1:5) in sequence, continue the heat preservation reaction for 20-24 hours, and monitor the reaction by TLC (developing agent: EA / PE=1 / 1, UV254nm, product Rf=0.5-0.8 or so) , cool down to 85-95°C, add water, stir to precipitate solids, slowly cool down ...
Embodiment 2
[0057] 1) Add ethanol, 2A6, water, and NaOH (molar ratio: 6:1.5:15:2.8) to the reaction kettle in sequence, heat to 75-85°C for 6-8 hours, and TLC detects that the raw material 2A6 has reacted completely ( Developing agent: EA:PE=1:1, UV254nm, product Rf=0). When the temperature is lowered to 60-70°C, add hydrochloric acid to adjust the pH to about 0.8-1.5, and solids are precipitated. Continue to cool down to 20-25°C and stir for 30-45 minutes. °C and dried under reduced pressure for 6-8 hours to obtain off-white powder 2A7.
[0058] 2) Add DMF, 2,5-bis(trifluoromethyl)aniline, DBU, CDI (molar ratio: 4:1.5:1.8) in turn to the reaction kettle under stirring, and heat to 105-110°C for 2.5-3 After 1 hour, add 2A7 and DMF (molar ratio: 1.2:4) in sequence, and continue the heat preservation reaction for 20-24 hours. TLC monitors the reaction (developing agent: EA / PE=1 / 1, UV254nm, product Rf=0.5-0.8 or so) , cool down to 85-95°C, add water, stir to precipitate solids, slowly cool...
Embodiment 3
[0061] Embodiment 3, comparative test
[0062] In order to investigate the optimal preparation method of dutasteride, the preparation method of the present invention was compared with the prior art,
[0063] Among them: Scheme 1: using toluene or directly pyridine as a solvent, using thionyl chloride or oxalyl chloride as an acid chloride reagent, through DT4-acyl chloride (III) and 2,5-bistrifluoromethylaniline (2,5- bis (trifluoromethyl)aniline, BTFMA) reaction method for preparing DTS (document WO, 95 / 07927);
[0064] Scheme 2: The DT4 acid chloride method is reacted with ammonia to produce DT4-amide (II), and BTFMA is diazotized to replace 2,5-bistrifluoromethyl iodobenzene (VI), both of which use xylene as a solvent. Catalyzed with potassium carbonate and copper powder, reflux reaction for 50-60 hours to prepare DTS (document US, 2005 / 0059692A1); scheme 3: using acetonitrile as solvent, DT4 is prepared with pivaloyl chloride or methanesulfonyl chloride under the catalysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com