Dutasteride soft capsule preparation and preparation process thereof
A technology of soft capsule preparation and dutasteride, which is applied in the direction of capsule delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of not being able to significantly improve the dissolution of soft capsules containing dutasteride Problems such as degree, influence on the stability and bioavailability of preparations, migration of active pharmaceutical compounds, etc., to achieve the effects of good product appearance quality, improved cumulative dissolution rate, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
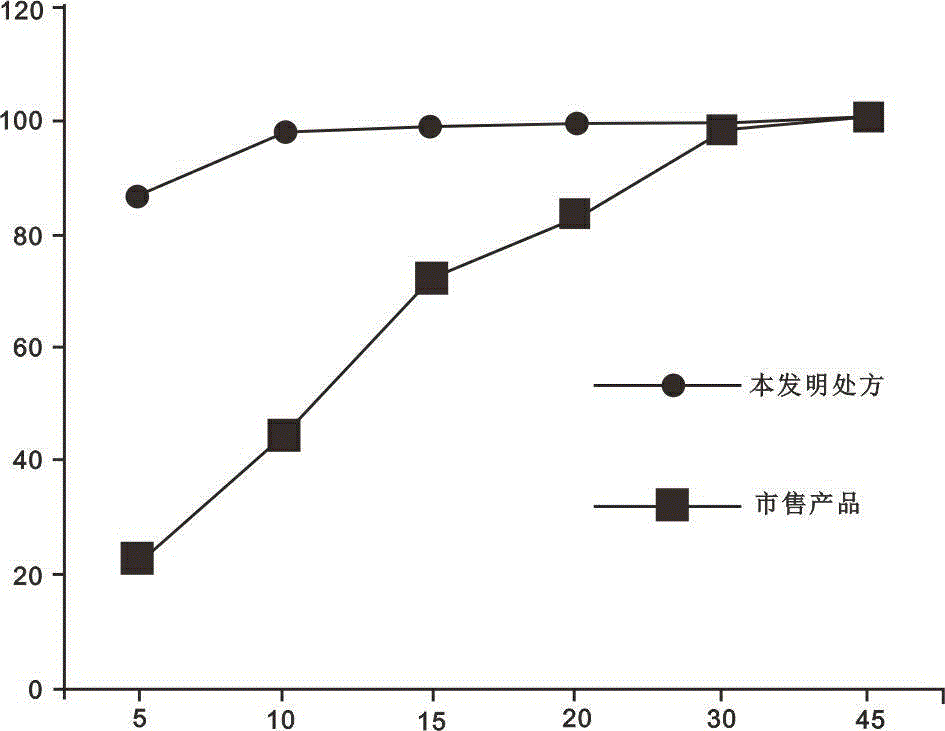
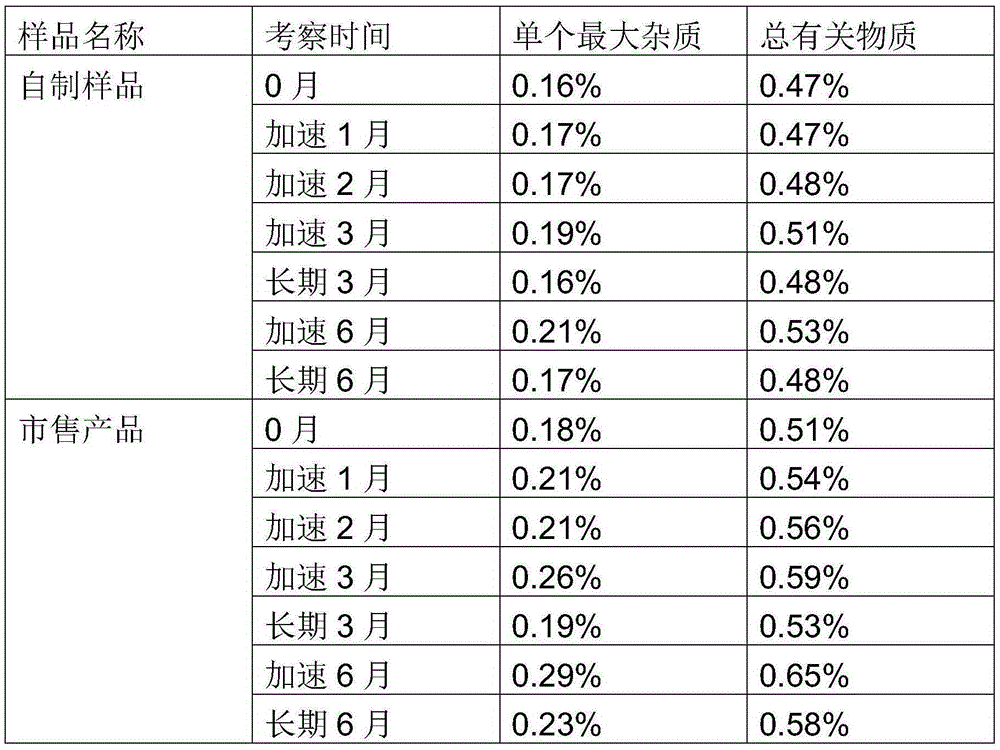
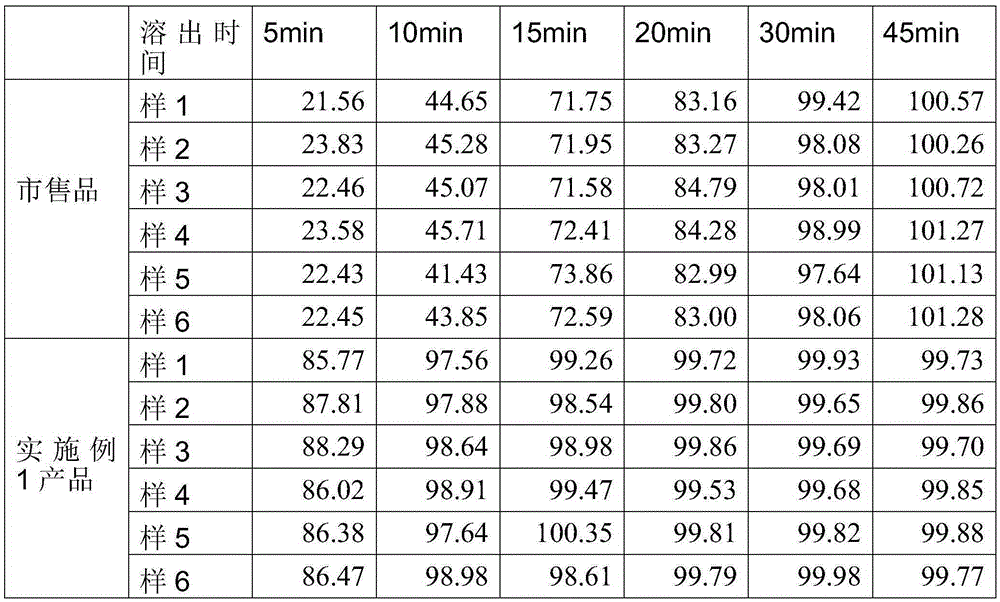
Image
Examples
Embodiment 1
[0057] Prescription of contents of dutasteride soft capsule preparation:
[0058] Dutasteride raw material 0.5g; caprylic capric acid macrogol glyceride 220g;
[0059] Polyglycerol oleate 45g; medium chain triglyceride 75g;
[0060] 0.035 g of 2,6-di-tert-butyl-p-cresol.
[0061] Softgel prescription:
[0062] Gelatin 160mg; Glycerin 85mg;
[0063] Yellow iron oxide 0.28mg; titanium dioxide 2mg.
[0064] Preparation:
[0065] (1) Preparation of contents: Take the prescription amount of caprylic capric acid macrogolglyceride, polyglycerol oleate and medium chain triglyceride and stir evenly, add the prescribed amount of dutasteride raw material after heating up to 40°C drug and 2,6-di-tert-butyl-p-cresol, continue to stir for 1h until uniform, and then cool down to room temperature to obtain the content;
[0066] (2) Preparation of soft capsules: Take the prescribed amount of glycerin, add water, put it in a plastic tank, stir and mix evenly, then add titanium dioxide and...
Embodiment 2
[0069] Prescription of contents of dutasteride soft capsule preparation:
[0070] Dutasteride raw material 0.5g; caprylic capric acid macrogol glyceride 225g;
[0071] Polyglycerol oleate 50g; medium chain triglyceride 72g;
[0072] 0.038g of 2,6-di-tert-butyl-p-cresol.
[0073] Softgel prescription:
[0074] Gelatin 165mg; Glycerin 83mg;
[0075] Yellow iron oxide 0.27mg; titanium dioxide 2.2mg.
[0076] Preparation:
[0077] (1) Preparation of contents: Take the prescription amount of caprylic capric acid macrogolglyceride, polyglycerol oleate and medium chain triglyceride and stir evenly, add the prescribed amount of dutasteride raw material after heating up to 40°C drug and 2,6-di-tert-butyl-p-cresol, continue to stir for 1h until uniform, and then cool down to room temperature to obtain the content;
[0078] (2) Preparation of soft capsules: Take the prescribed amount of glycerin, add water, put it in a plastic tank, stir and mix evenly, then add titanium dioxide and ...
Embodiment 3
[0081] Prescription of contents of dutasteride soft capsule preparation:
[0082] Dutasteride raw material 0.5g; caprylic capric acid macrogol glyceride 220g;
[0083] Polyglycerol oleate 45g; medium chain triglyceride 75g;
[0084] Softgel prescription:
[0085] Gelatin 160mg; Glycerin 85mg;
[0086] Yellow iron oxide 0.28mg; titanium dioxide 2mg.
[0087] Preparation:
[0088] (1) Preparation of contents: Take the prescription amount of caprylic capric acid macrogolglyceride, polyglycerol oleate and medium chain triglyceride and stir evenly, add the prescribed amount of dutasteride raw material after heating up to 40°C Continue to stir the medicine for 1 hour until it is uniform and then cool down to room temperature to obtain the content;
[0089] (2) Preparation of soft capsules: Take the prescribed amount of glycerin, add water, put it in a plastic tank, stir and mix evenly, then add titanium dioxide and yellow iron oxide passed through an 80-mesh sieve and mix evenl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com