Synthesis method of dutasteride intermediate
A synthetic method, dutasteride technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of high price, high cost, unsuitable for large-scale industrial production, etc., to achieve increased yield, improved safety, reduced cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
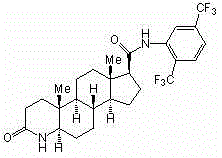
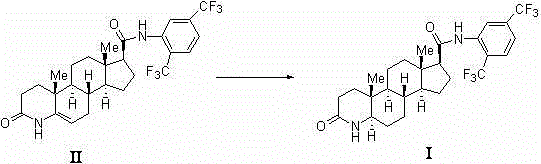
[0038] Compound Ⅱ (25 g, 47 mmol), methanol (1000 mL), 2 mol / L HCl aqueous solution to adjust pH=2, add NaBH 3 CN (5.9g, 94mmol), stirred at room temperature for 1 h, then adjusted pH=2 with 2 mol / L HCl aqueous solution, added water (100 mL), adjusted pH=8 with 10% NaOH solution, CH 2 Cl 2 Extraction, the organic layer was dried with anhydrous magnesium sulfate, filtered, the filtrate was evaporated to dryness under reduced pressure, a small amount of ethyl acetate was added to the crude product and heated to reflux, and ethyl acetate was continuously added to dissolve it completely, cooled at room temperature and left standing to obtain a white crystalline solid compound Ⅰ (18g, 72%), the purity was over 98.0% by HPLC, m.p. 245~247℃.
Embodiment 2
[0040] Compound Ⅱ (25 g, 47 mmol), methanol (1000 mL), 2 mol / L HCl aqueous solution to adjust pH=2, add NaBH 3 CN (12.6 g , 201 mmol), stirred at room temperature for 1 h. Then use 2 mol / L HCl aqueous solution to adjust pH=2, add water (100 mL), adjust pH=8 with 10% NaOH solution, CH 2 Cl 2 Extraction, the organic layer was dried with anhydrous magnesium sulfate, filtered, the filtrate was evaporated to dryness under reduced pressure, a small amount of ethyl acetate was added to the crude product and heated to reflux, and ethyl acetate was continuously added to dissolve it completely, cooled at room temperature and left standing to obtain a white crystalline solid compound Ⅰ (23 g, 92%), the purity was over 98.0% by high performance liquid chromatography, m.p. 245~247℃.
Embodiment 3
[0042]Compound Ⅱ (25 g, 47 mmol), methanol (1000 mL), 2 mol / L HCl aqueous solution to adjust pH=2, add NaBH 3 CN (23.6 g, 376 mmol), stirred at room temperature for 1 h. Then use 2 mol / L HCl aqueous solution to adjust pH=2, add water (100 mL), adjust pH=8 with 10% NaOH solution, CH 2 Cl 2 Extraction, the organic layer was dried with anhydrous magnesium sulfate, filtered, the filtrate was evaporated to dryness under reduced pressure, a small amount of ethyl acetate was added to the crude product and heated to reflux, and ethyl acetate was continuously added to dissolve it completely, cooled at room temperature and left standing to obtain a white crystalline solid compound Ⅰ (21g, 84%), the purity was over 98.0% by HPLC, m.p. 245~247℃.
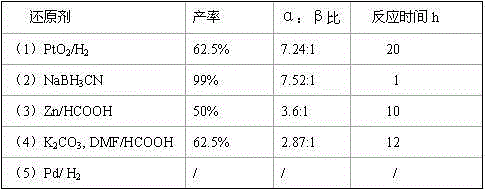
[0043] From the results of Examples 1 to 3, it can be seen that when compound II and reducing agent NaBH 3 When the molar ratio of CN is 1:4, the yield of the synthesis reaction is the highest, which is the best choice.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com