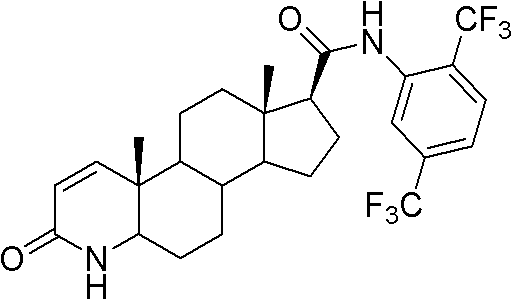
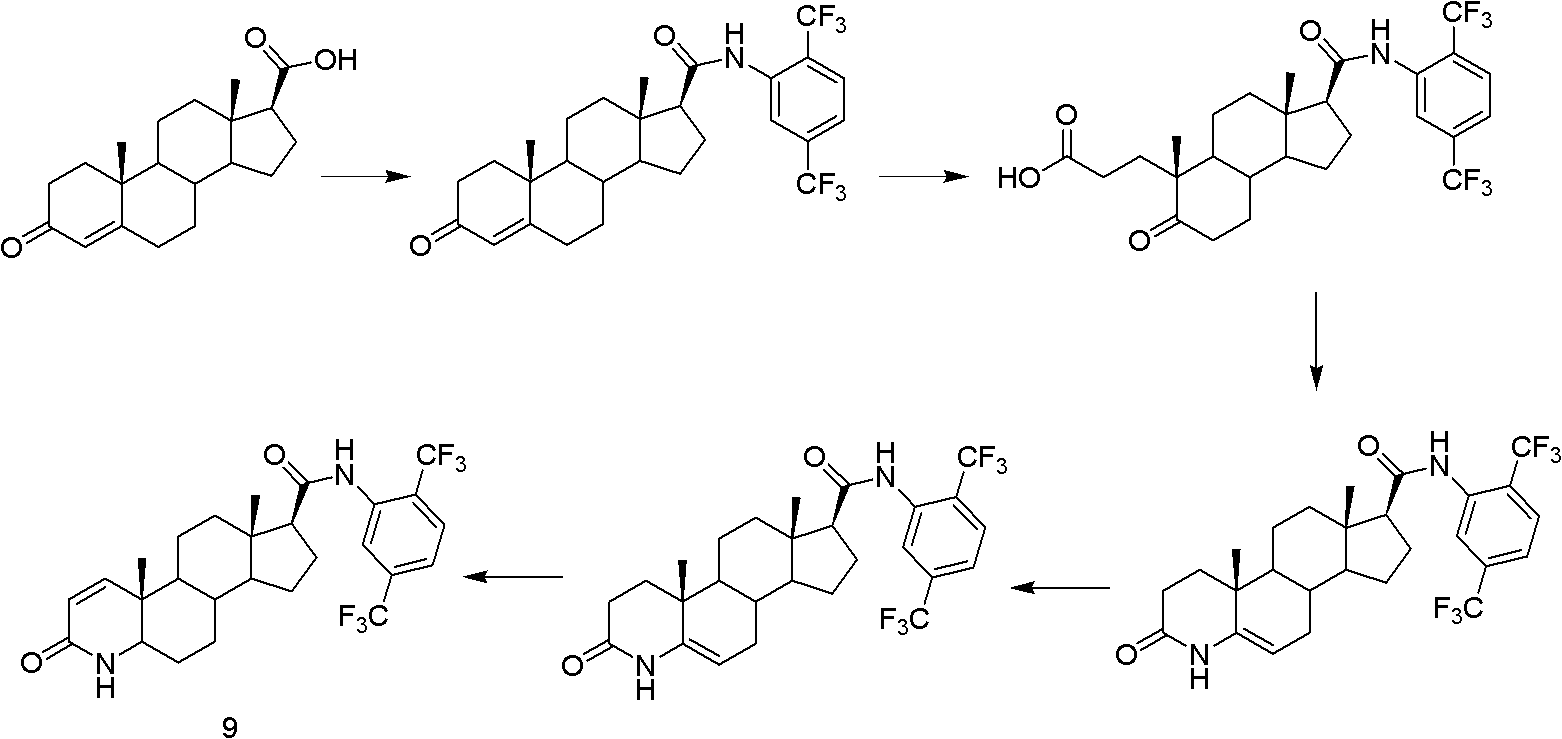
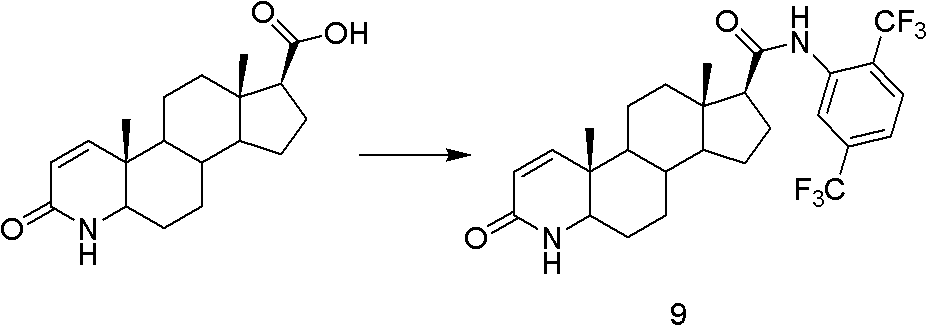
Method for preparing dutasteride
A technology of dutasteride and aniline, which is applied in the field of preparation of chemical drugs, can solve problems such as personal danger of operators, achieve the effects of reducing costs, saving steps, and improving process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Dissolve 10.0g of androstenedione in 220ml of tert-butanol, add 50ml of an aqueous solution containing 9g of anhydrous potassium carbonate, heat up to 45°C, dropwise add 160ml of an aqueous solution containing 20g of potassium permanganate and 20g of sodium periodate, add Continue the reaction for 0.5 hours, cool to room temperature, filter, remove tert-butanol under reduced pressure, cool, adjust pH to 1-2 with concentrated hydrochloric acid, extract with dichloromethane, dry the organic phase with anhydrous sodium sulfate, and concentrate under reduced pressure A white solid compound 2 was obtained, which was dried to obtain 8.9 g. IR(KBr)(cm -1 ): 3048-3503, 1730, 1706.
Embodiment 2
[0044] 8.5g of compound (2), 26g of ammonium acetate, and 50ml of acetic acid solution were added to a three-necked flask equipped with a reflux condenser, refluxed at 105°C for 3h, cooled, added with 50ml of water, reacted for 12h, filtered, washed with water, and dried in vacuo to obtain compound ( 3) 6.3g. IR(KBr)(cm -1 ): 3185, 3065, 1739, 1675, 1660.
Embodiment 3
[0046] 6 g of compound (3) was dissolved in tetrahydrofuran, and 50 ml of 1.7M methylmagnesium iodide solution in tetrahydrofuran was slowly added dropwise. It was dried over anhydrous sodium sulfate and concentrated to obtain 6.3 g of compound (4). IR(KBr)(cm -1 ) 3429, 3150, 1748, 1683. ESI-MS m / z (%): [M+H] + =303.4; 1H NMR (DMSO) δ: 0.83 (s, 3H, 8-CH 3 ), 0.95(s, 3H, 19-CH 3 ), 1.08(s, 3H, 20-CH 3 ), 3.3 (m, 1H, 5α-H), 7.62 (1H.NH), 8.05 (br.1H.OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com